Abstract
Background
Idiopathic pulmonary fibrosis (IPF) is the most common idiopathic interstitial disease of the lung and has the worst prognosis of all such diseases, with a median survival time of three to four years. Its prevalence is 2–29 per 100 000 persons and its incidence approximately 10 per 100 000 persons per year, with an upward trend.
Methods
Selective literature search in the EMBASE and PubMed databases for pertinent publications from 1996 to 2012, with special attention to randomized controlled trials
Results
IPF manifests itself clinically with exertional dyspnea, dry cough, and inspiratory crepitations (sclerosiphonia). The diagnosis is confirmed by the demonstration of a usual interstitial pneumonia (UIP) pattern in a high-resolution thin-slice CT (HRCT) of the lungs, or else histologically by lung biopsy, along with the exclusion of other causes such as asbestosis or connective tissue disease. In 15 randomized controlled therapeutic trials carried out since 2004, most of the drugs that were tested, including immune suppressants, were found to be ineffective against IPF or even harmful. Only pirfenidone lessens the annual reduction of pulmonary volume (FVC, forced expiratory vital capacity) and of the distance walked in 6 minutes by about 30%, with corresponding improvement of progression-free survival, but without any significant lessening of overall mortality (placebo, 10%; pirfenidone, 8%). Pirfenidone also commonly causes gastrointestinal and cutaneous side effects. The efficacy of N-acetyldysteine and nintedanib has not yet been definitively demonstrated. Lung transplantation is the only current treatment that enables long-term survival.
Conclusion
IPF has a worse prognosis than many types of cancer. Drugs can delay the progression of the disease but probably cannot bring it to a permanent standstill.
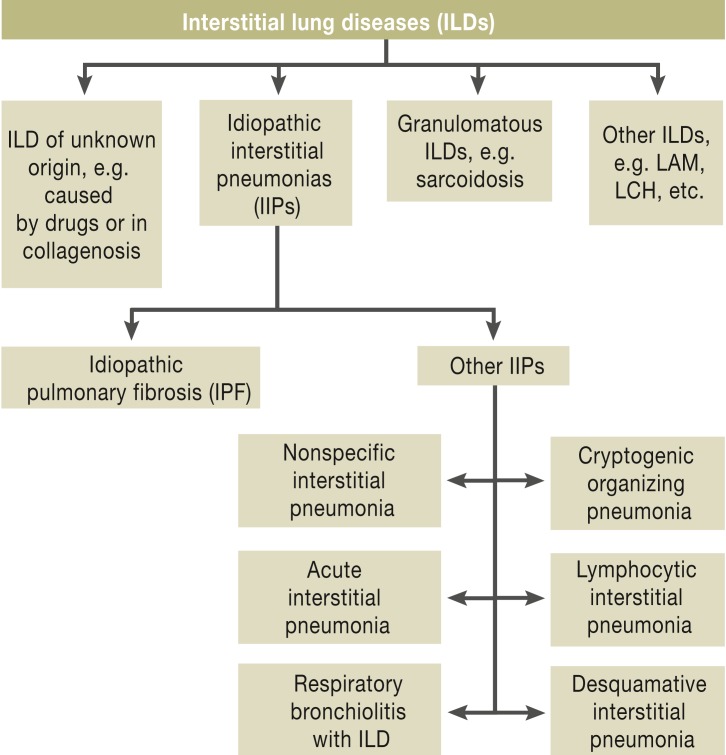
The first modern classification system for lung diseases was developed by Liebow, Carrington, and Gaensler in the 1960s and 1970s. Even this first classification described usual interstitial pneumonia (UIP) as particularly aggressive (1). In 2002, the American Thoracic Society and the European Respiratory Society published a comprehensive classification system (figure 1) (2). The histological pattern of UIP was recognized as being characteristic of the clinical picture of idiopathic pulmonary fibrosis (IPF), which is progressive, fatal fibrotic interstitial lung disease of unknown origin.
Figure 1.
Classification of interstitial lung diseases (2)
LAM: lymphangioleiomyomatosis; LCH: Langerhans cell histiocytosis; ILD: Interstitial lung disease; IIPs: Idiopathic interstitial pneumonias; IPF: Idiopathic pulmonary fibrosis;
New knowledge of the etiology and pathogenesis of IPF, technical advances in radiological diagnostics, and new treatment approaches have led to a revision of the existing guidelines (3– 5). These factors are described below.
Methods
This article is based on a selective search of the literature in EMBASE and PubMed (search period 1996 to 2012). The search involved the following search terms, in varying combinations: “human,” “fibrosis,” “pulmonary,” “lung,” “idiopathic,” “pathogenesis,” “clinical trials,” “treatment outcome,” “random.”
Definition
IPF is chronic, progressive, fibrotic interstitial pneumonia of unknown origin, usually affecting older people. It is restricted to the lungs and associated with a histopathological and/or radiological pattern of UIP (4, 5). A diagnosis of IPF requires that other forms of interstitial lung disease are ruled out.
Epidemiology
IPF affects older adult patients with a peak prevalence around age 65 years. With a prevalence of 2 to 29 per 100 000 and an incidence of approximately 10 per 100 000/year, IPF meets the criteria for classification as an orphan disease. However, it is up to 10 times more prevalent in population groups aged over 70 years as compared to the mean prevalence in the total population (6, 7). The median survival time following diagnosis of IPF is 3 to 4 years; its mortality rate is therefore higher than that of most cancers (e1). Patient numbers are rising overall; it is impossible to tell whether this is merely a reflection of demographic change and improved diagnosis or a real increase in the frequency of IPF (7). The diagnosis and treatment of IPF poses a particular challenge in older patients, as a result of comorbidities.
Etiology, pathogenesis
Although the cause of IPF is unknown, the results of recent research have shed some light on the matter. For example, in cases of familial and sporadic pulmonary fibrosis there was evidence of mutations of the genes for surfactant proteins A and C, telomerase complex mutations, and single-nucleotide polymorphism in the MUC5B promoter. These are causally associated with IPF (4, 5, e2, e3).
Regarding the pathogenesis of IPF, it is currently accepted that alveolar epithelial cell apoptosis leads to the following processes:
Disruption to regeneration
Fibroblast activation
Differentiation to myofibroblasts, with increased proliferation
Extracellular matrix deposition.
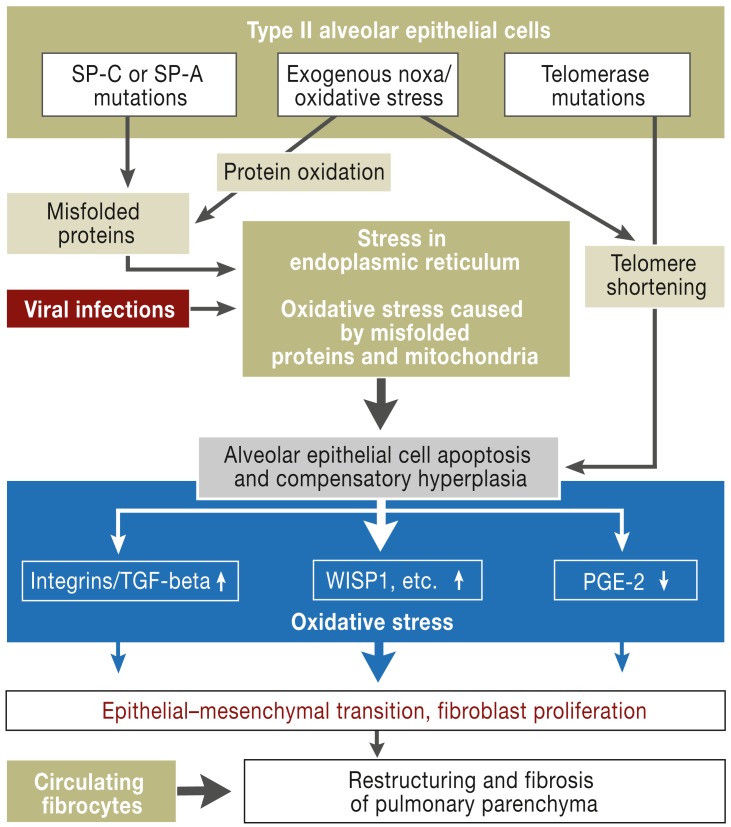
Together with the aging process, exogenous factors observed in association with IPF, such as viral infections, inhaled toxins (e.g. cigarette smoke), and microaspiration of gastric acid, can trigger alveolar epithelial cell apoptosis and thereby disease manifestation and progression (4, 5). figure 2 shows a current view of the pathogenesis of IPF.
Figure 2.
Pathogenesis of idiopathic pulmonary fibrosis
SP-C/A: surfactant protein C/A; TGF-beta: transforming growth factor–beta;
WISP: wingless/int-1 (Wnt)–induced secreted protein-1; PGE2: prostaglandin E2
Disease progression
IPF progresses heterogeneously. The best method of determining progression is to identify a decline in FVC (forced vital capacity). Regular lung function checks to measure vital and diffusing capacity are therefore recommended at intervals of three to six months (5). Functionally stable patients show decline in FVC of a maximum of 5% of the target value over 6 to 12 months (8– 10). A decline in FVC of 5 to 10 percentage points indicates prognostically relevant progression, while a decline of 10 percentage points or more in six months is associated with a fourfold to eightfold increase in the risk of death in the next 12 months (8– 10).
Acute exacerbations are an important aspect of the clinical progression of IPF. These are characterized by an increase in shortness of breath over a period of 30 days and a high-resolution computed tomography (HRCT) showing new infiltration of the lungs with no identifiable cause. Their frequency is 5 to 15% per year and they are associated with high mortality. Following an acute exacerbation of IPF approximately 50% of patients die within three months, and approximately 80 to 90% within 12 months (11).
Diagnosis
The onset of clinical symptoms is insidiuos. Symptoms include shortness of breath on exertion and a dry cough. Some patients report an initial, flu-like malaise.
On physical examination, auscultation almost always (in ≥90% of cases) reveals inspiratory crepitations, particularly basally and basolaterally, i.e. in the areas of greatest respiratory movement (e4). This finding should always be further explored via lung function testing that includes measurement of diffusion and radiologic imaging.
Further clinical signs are hippocartic nails (in approximately 50% of cases) and finger clubbing (approximately 20%) (4, 5). In advanced cases signs include cyanosis, dyspnea at rest, and signs of right-sided ventricular stress.
Lung function testing, including spirometry, whole-body plethysmography, and measurement of diffusion, can be used to identify restricted lung volume and abnormal gas exchange objectively. It can also assess the severity of IPF, with the resulting implications for prognosis. However, normal lung function does not rule out incipient IPF.
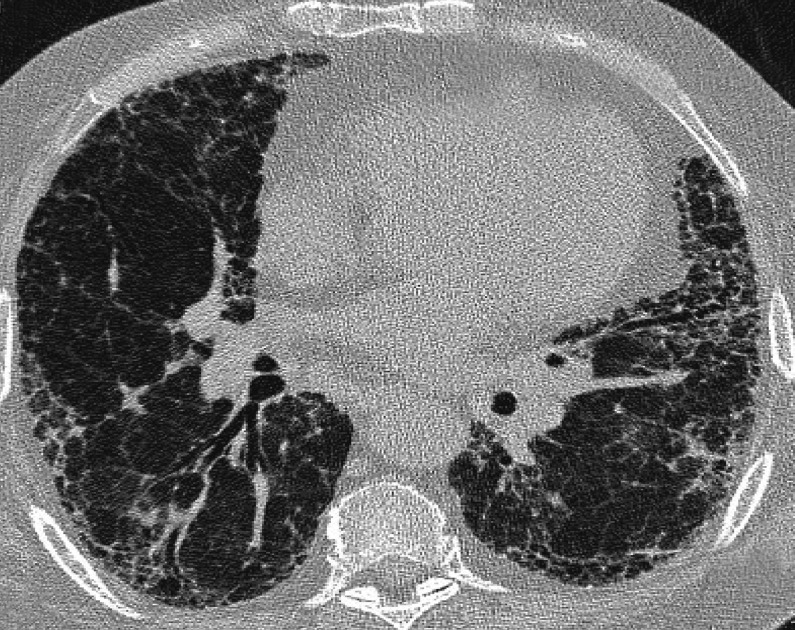
High-resolution computed tomography (HRCT) (slice thickness ≤ 2.0 mm, no contrast media) should always be performed to confirm clinically suspected interstitial lung disease (4, 5). The radiological criteria for a UIP pattern are subpleurally and basally predominant increased interstitial striation with honeycombing and sometimes traction bronchiectasis (dilated bronchi caused by shrinkage of the surrounding lung tissue) (4, 5). There must also be an absence of extensive ground-glass opacities, consolidation, cysts, granulomas, or microgranulomas (4, 5). figure 3 shows an example of a typical UIP pattern on HRCT.
Figure 3.
Typical UIP pattern on HRCT.
HRCT: high-resolution computed tomography; UIP: usual interstitial pneumonia
If HRCT shows a definite UIP pattern and no cause for the pulmonary alterations can be identified, IPF can be diagnosed. The differential diagnosis of IPF is extensive and difficult. It should take into account both a detailed medical history—including occupational exposures (e.g. asbestos), private exposures (e.g. bird ownership), and a comprehensive drug history—and immunological biomarkers (rheumatoid factor, cyclic citrullinated peptide, and antinuclear antibodies) (5). The value of bronchioalveolar lavage (BAL), the results of which aid differential diagnosis (12), is rated differently by different parties internationally. For example, in international guidelines it is not generally recommended, while the German guidelines on IPF do recommend BAL for all patients with a UIP pattern as the latter may be mimicked by chronic hypersensitivity pneumonitis, which would show up as lymphocytosis on BAL (4, 5, 12). The less conclusive the UIP pattern, the more important is differential diagnosis of IPF versus other interstitial lung diseases; this justifies the use of BAL and transbronchial lung biopsy.
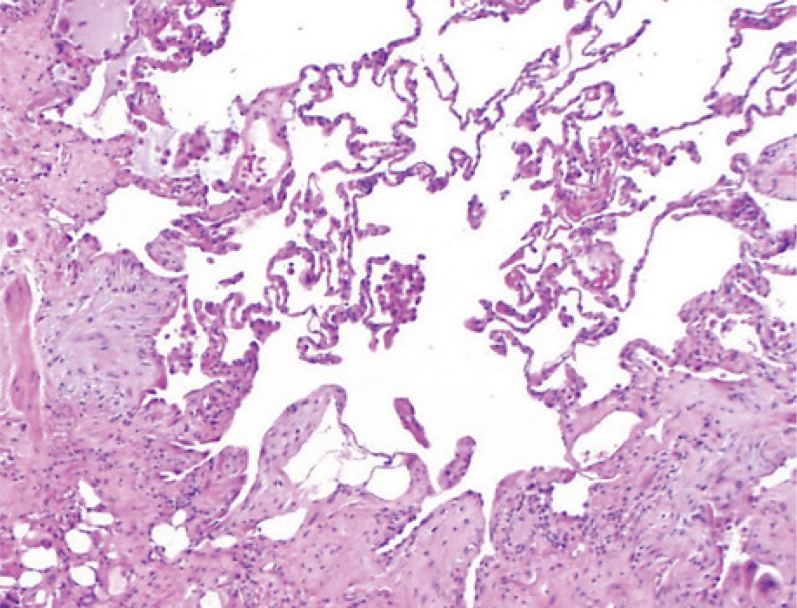
If HRCT does not reveal a conclusive UIP pattern, surgical lung biopsy is indicated. This usually takes the form of video-assisted thoracoscopic (VATS) lung biopsy. The histological criteria or a UIP pattern include a heterogeneous picture of normal alveolar areas adjacent to fibrotically thickened alveolar septa, and honeycombing. Cellular inflammatory response is low or absent, as are granulomas, organizing pneumonia, and hyaline membrane disease (4, 5). figure 4 shows the typical histology of the UIP pattern.
Figure 4.
Typical histological UIP pattern.
UIP: Usual interstitial pneumonia
Use of surgical lung biopsy is limited by its complications, which include persistent bronchopleural fistulas and perioperative lung failure in the context of an acute exacerbation of IPF (13, 14). Patients whose vital capacity or diffusion capacity is already severely restricted (<55% of target value, <40% of target value in single-breath testing respectively) and those receiving long-term oxygen therapy have a significantly increased risk of mortality. Particularly thorough risk/benefit analysis is therefore essential (13, 14). Bronchoscopic transbronchial forceps biopsy is not sufficient for diagnosis. Transbronchial cryobiopsy is currently in use in experienced centers; whether it can provide sufficient material at a low risk is currently being investigated in a controlled, multicenter study.
Studies on interobserver variability have shown that there are considerable discrepancies between the conclusions of even specialist examiners when evaluating both HRCT and histology. For example, approximately 10% of IPF patients in clinical studies turn out to actually suffer from chronic hypersensitivity pneumonitis (15– 19, 26). Diagnosis is most reliable if it results from interdisciplinary discussion of all findings by pulmonologists, radiologists, and pathologists, although even this results in an ultimate diagnosis of “unclassifiable fibrosis” in approximately 10% of cases (4, 5, 15, 20).
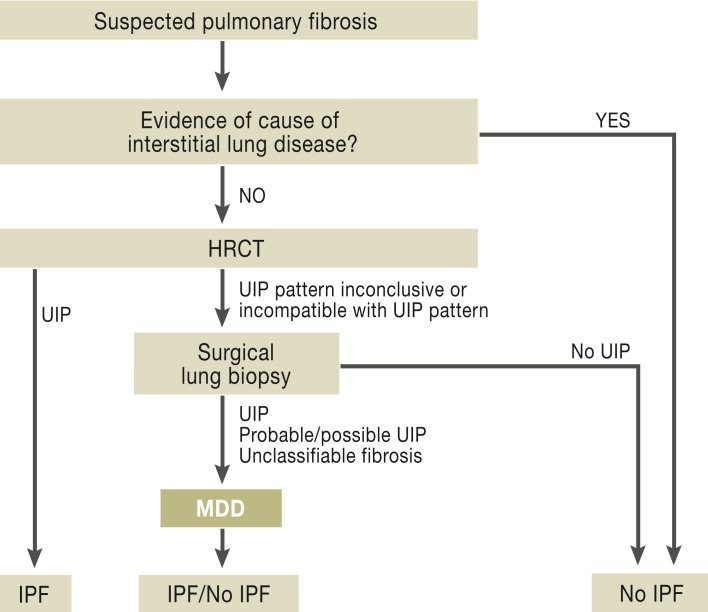
Figure 5 shows the diagnostic algorithm in cases of clinically suspected pulmonary fibrosis. In view of the complexity of diagnosis and its impact on prognosis, patients should be referred to a center or pulmonary specialist with expertise in interstitial lung diseases for initial diagnosis.
Figure 5.
IPF diagnosis procedure (4, 5)
HRCT: high-resolution computed tomography; UIP: usual interstitial pneumonia; MDD: multidisciplinary discussion; IPF: idiopathic pulmonary fibrosis
Pharmacological treatment
The last 15 years have seen a fundamental change in IPF treatment. The earlier use of anti-inflammatory treatment, involving prednisolone and azathioprine, was based on the premise that chronic inflammation of the alveoli (alveolitis) triggered and promoted fibrosis. However, this “alveolitis hypothesis” was shown to be untrue of IPF, and the treatment was not only ineffective but even potentially harmful, as shown in the recently published PANTHER study (21). This was a double-blind randomized trial in which IPF patients received combined prednisolone, azathioprine, and N-acetylcysteine therapy (triple therapy) and were compared to a placebo group. After a mean duration of 32 weeks mortality was already significantly increased in the intervention arm, and the study had to be terminated early (21). This triple therapy, once used frequently, is therefore no longer recommended for patients with definite IPF (21– 23). There are, however, various forms of other fibrotic interstitial lung diseases, other than IPF, in which inflammatory disease mechanisms play a prominent role; for these, anti-inflammatory and immunosuppressant drugs, including triple therapy with prednisolone, azathioprine, and N-acetylcysteine, are effective treatments (22, 23). Differential diagnosis of idiopathic pulmonary fibrosis versus other diseases before treatment is initiated is therefore essential.
Antifibrotic treatment options were developed on the basis of new knowledge regarding the pathogenesis of IPF and negative experiences with anti-inflammatory therapy. However, in Phase II and III clinical trials several drugs with mechanisms of action that include antifibrotic effects have proved ineffective (interferon gamma-1b, endothelin receptor antagonists, TNF-alpha antagonists, PDGF inhibitors) or even harmful (anticoagulants) (24– 30). One exception is pirfenidone, an orally available pyridone derivative that exhibits antifibrotic, anti-inflammatory, and antioxidant effects both in vitro and in vivo, although no specific mechanism of action has yet been identified. Pirfenidone was first used in IPF patients in an open-label observation study in 1999, but treatment efficacy could not be reliably evaluated as there was no control group (31). Next came Phase II and III trials in Japan; these found positive effects on lung function and progression-free survival, leading to the licensing of pirfenidone in Japan in 2008 (32, 33). Two parallel, similarly-designed Phase III trials of pirfenidone treatment for IPF (CAPACITY 1 and CAPACITY 2) were conducted using the same endpoints in North America, Australia, and Europe (34).
Both these trials yielded ambiguous results in terms of statistical significance, but they did show the same trends in treatment effects in terms of the most important endpoints. Pooled data analysis was therefore performed (34). Decline in FVC up to week 72 as a percentage of the target value (the primary endpoint) was significantly lower, at –8.5%, for pirfenidone (2403 mg/d) than for placebo (–11%) (p = 0.005) (34). 87 patients were randomized to receive a lower dose of pirfenidone, 1197 mg/d, and showed an intermediate response in terms of decline in FVC when compared to patients receiving the full dose of 2403 mg/day (34). With the full dose of 2403 mg/day the six-minute walk distance (6MWD) decreased by a mean of 24 m less (placebo: decrease from 405 m to 328 m; pirfenidone 395 m to 342 m; p = 0.0009), while progression-free survival (PFS) over 72 weeks was 26% better (p = 0.025) (34).
An independent Cochrane meta-analysis found a significant effect on PFS in the CAPACITY trials and the Japanese Phase III trial, with a relative risk reduction of 30% (35). The total mortality rate observed in the CAPACITY trials showed a favorable trend for pirfenidone (pirfenidone 27/345 versus placebo 34/347, p = 0.315) (34). This trend was even stronger for death from IPF (18/345 versus 28/347), but still not statistically significant (p = 0.117) (34). Positive evaluation of the efficacy of pirfenidone is supported by trials that show a significant correlation between decline in FVC and mortality and between decrease in 6MWD and mortality (9, 10, 36). However, the side effects of pirfenidone—nausea, vomiting and loss of appetite, dizziness, increased liver enzymes, skin alterations up to severe phototoxic reactions—should not be overlooked (34). Patients receiving pirfenidone therapy must therefore routinely use sun block (sun protection factor 50) on areas of skin exposed to the sun when leaving the house (31– 34). In the licensing study the drop-out rate due to side effects was 15% for pirfenidone and 9% for placebo (34).
On the basis of these trials, pirfenidone was licensed by the European drug authorities for the treatment of mild and moderate IPF in February 2011. In contrast, in October 2010 the US FDA refused to license it and demanded further trials: one (the ASCEND trial) is currently ongoing in the USA.
The German guidelines on IPF contain a weak positive recommendation for the use of pirfenidone in patients with mild to moderate IPF (5). The phrase “weak positive recommendation” reflects the persisting uncertainty regarding its degree of efficacy—it delays disease progression but may not halt it in the long term—and its potential side effects. As a result, in each individual case the benefits and side effects must be critically considered and the patient must be carefully informed before treatment is initiated.
Numerous other therapeutic approaches are currently being investigated in Phase III trials:
Antioxidant therapy with high-dose N-acetylcysteine (NAC), derived from the results of the IFIGENIA study (37), is being evaluated in the placebo-controlled PANTHER IPF study (21, 37).
The multikinase inhibitor nintedanib showed positive effects on decline in FVC and the number of acute exacerbations in a Phase II trial (38).
A pilot study found a favorable effect on lung function and HRCT for monoclonal antibody to connective tissue growth factor (anti-CTGF antibody) (39).
Comorbidities
In addition to targeted antifibrotic treatment of the underlying disease, treatment of comorbidities also plays an important role. For example, treating severe pulmonary hypertension or sleep apnea can improve the condition of individual patients (e5– e9). Strict indication criteria should be applied for the treatment of pulmonary hypertension, and efforts should be made to achieve evidence of a response to treatment in the individual patient (40). Approximately 25% of IPF patients have an additional disorder of left ventricular function that requires treatment (e10). Gastro-esophageal reflux (GER) is observed in up to 80% of IPF patients; it is being discussed as a trigger for disease progression and acute exacerbations (e11, e12). GER should therefore be routinely treated in IPF patients (4, 5). Reactive depression requiring treatment is often observed in IPF patients (e13). In advanced stages of the disease, where there is no prospect of transplantation, timely palliative care should be initiated and end-of-life scenarios should be discussed with patients and their relatives (5).
Nonpharmacological treatment
To date, the only known treatment for IPF that provides a chance of long-term survival is lung transplantation (4, 5). However, this can only be considered for a small proportion of patients, due to the age distribution on the one hand and frequent comorbidities on the other. IPF patients under 65 years of age with no contraindications should be referred to a lung transplantation center—at the latest when there is hypoxemia at rest ≤55 mg Hg, desaturation on exertion (SpO2 <89% in 6MWT), or disease progression (e.g. decline in FVC >10% over six months) (4, 5). Further measures include long-term oxygen therapy when there is evidence of hypoxemia ≤55 mm Hg at rest, and pulmonary rehabilitation (4, 5, e14, e15).
Key Messages.
IPF is a rare disease but is more common in older people.
With a median survival time of 3 to 4 years, IPF has a worse prognosis than most cancers. Only lung and pancreatic cancer have a worse five-year survival rate, less than 20%.
Clinical findings include dyspnea, while auscultation reveals basal crepitations. This should always be explored further.
Diagnosis requires radiological (HRCT) or histological evidence of a UIP pattern. Known causes of pulmonary fibrosis must be ruled out.
The efficacy of drug treatment with pirfenidone for IPF is unsatisfactory, but a delay in disease progression of approximately 30% is both possible and worthwhile. The efficacy of N-acetylcysteine and nintedanib cannot yet be conclusively evaluated but is probably comparable based on the available data.
The most important nonpharmacological treatment approaches to IPF are long-term oxygen therapy, pulmonary rehabilitation, and lung transplantation.
Footnotes
Conflict of interest statement
Prof. Behr has received consultancy fees from Actelion, Bayer, Boehringer, InterMune, GSK, Novartis, Roche, and Zambon. He has received reimbursement of conference fees and travel expenses from Boehringer, Actelion, and Intermune. He has received lecture fees from Actelion, Bayer, Boehringer, InterMune, and Zambon.
References
- 1.Behr J, Costabel U. Interstitial lung diseases—historical development, current status, future prospects. Pneumologie. 2010;64:573–576. doi: 10.1055/s-0030-1255627. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society, European Respiratory Society. International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society, European Respiratory Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis Anofficial ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behr J, Günther A, Ammenwerth W, et al. S2K-Leitlinie zur Diagnostik und Therapie der idiopathischen Lungenfibrose. Pneumologie. 2013:;67:81–111. doi: 10.1055/s-0032-1326009. [DOI] [PubMed] [Google Scholar]
- 6.Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 7.Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev. 2012;21126:355–361. doi: 10.1183/09059180.00002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zappala CJ, Latsi PI, Nicholson AG, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:830–836. doi: 10.1183/09031936.00155108. [DOI] [PubMed] [Google Scholar]
- 9.du Bois RM, Weycker D, Albera C, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:459–466. doi: 10.1164/rccm.201011-1790OC. [DOI] [PubMed] [Google Scholar]
- 10.du Bois RM, Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med. 2011;184:1382–1389. doi: 10.1164/rccm.201105-0840OC. [DOI] [PubMed] [Google Scholar]
- 11.Collard HR, Moore BB, Flaherty KR, et al. Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators: Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohshimo S, Bonella F, Cui A, Beume M, Kohno N, Guzman J, Costabel U. Significance of bronchoalveolar lavage for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:1043–1047. doi: 10.1164/rccm.200808-1313OC. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto S, Homma S, Mun M, Fujii T, Kurosaki A, Yoshimura K. Acute exacerbation of idiopathic interstitial pneumonia following lung surgery in 3 of 68 consecutive patients: a retrospective study. Intern Med. 2011;50:77–85. doi: 10.2169/internalmedicine.50.3390. [DOI] [PubMed] [Google Scholar]
- 14.Bando M, Ohno S, Hosono T, et al. Risk of acute exacerbation after video-assisted thoracoscopic lung biopsy for interstitial lung disease. J Bronchology Interv Pulmonol. 2009;16:229–235. doi: 10.1097/LBR.0b013e3181b767cc. [DOI] [PubMed] [Google Scholar]
- 15.Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164:193–196. doi: 10.1164/ajrccm.164.2.2101090. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson AG, Fulford LG, Colby TV, du Bois RM, Hansell DM, Wells AU. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166:173–177. doi: 10.1164/rccm.2109039. [DOI] [PubMed] [Google Scholar]
- 17.Flaherty KR, Thwaite EL, Kazerooni EA, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax. 2003;58:143–148. doi: 10.1136/thorax.58.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aziz ZA, Wells AU, Hansell DM, et al. HRCT diagnosis of diffuse parenchymal lung disease: inter-observer variation. Thorax. 2004;59:506–511. doi: 10.1136/thx.2003.020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomeer M, Demedts M, Behr J, et al. Idiopathic Pulmonary Fibrosis International Group Exploring N-Acetylcysteine I Annual (IFIGENIA) study group Multidisciplinary interobserver agreement in the diagnosis of idiopathic pulmonary fibrosis. Eur Respir J. 2008;31:585–591. doi: 10.1183/09031936.00063706. [DOI] [PubMed] [Google Scholar]
- 20.du Bois RM. An earlier and more confident diagnosis of idiopathic pulmonary fibrosis. Eur Respir Rev. 2012;21:141–146. doi: 10.1183/09059180.00000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Idiopathic Pulmonary Fibrosis Clinical Research Network. Prednisone, Azathioprine, and N-Acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366:1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behr J, Costabel U, Worth H. Comments of the DGP on the press release dated 21.10.2011 of the National Heart, Lung and Blood Institute about the PANTHER Study on IPF patients. Pneumologie. 2011;65:724–725. doi: 10.1055/s-0031-1291535. [DOI] [PubMed] [Google Scholar]
- 23.Wells AU, Behr J, Costabel U, Cottin V, Poletti V. Triple therapy in idiopathic pulmonary fibrosis: an alarming press release. Eur Respir J. 2012;39:805–806. doi: 10.1183/09031936.00009112. [DOI] [PubMed] [Google Scholar]
- 24.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE. Idiopathic Pulmonary Fibrosis Study Group A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350:125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 25.King TE Jr, Albera C, Bradford WZ, et al. INSPIRE Study Group. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet. 2009;374:222–228. doi: 10.1016/S0140-6736(09)60551-1. [DOI] [PubMed] [Google Scholar]
- 26.King TE Jr, Behr J, Brown KK, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 27.King TE Jr, Brown KK, Raghu G, et al. BUILD-3: A Randomized, Controlled Trial of Bosentan in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2011;184:92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 28.Raghu G, Brown KK, Costabel U, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178:948–955. doi: 10.1164/rccm.200709-1446OC. [DOI] [PubMed] [Google Scholar]
- 29.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR. Imatinib-IPF Study Investigators Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 30.Noth I, Anstrom SB, de Andrade J, Flaherty KR, Glazer C, Kaner RJ. Olman MA and the Idiopathic Pulmonary Fibrosis Clinical Research Network (IPFnet): A Placebo-Controlled Randomized Trial of Warfarin in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2012;186:88–95. doi: 10.1164/rccm.201202-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999;159:1061–1069. doi: 10.1164/ajrccm.159.4.9805017. [DOI] [PubMed] [Google Scholar]
- 32.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone Clinical Study Group in Japan Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 34.Noble PW, Albera C, Bradford WZ, et al. CAPACITY Study Group Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 35.Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Wlaters EH, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010;9 doi: 10.1002/14651858.CD003134.pub2. CD003134. [DOI] [PubMed] [Google Scholar]
- 36.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med. 2011;183:1231–1237. doi: 10.1164/rccm.201007-1179OC. [DOI] [PubMed] [Google Scholar]
- 37.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, et al. IFIGENIA Study Group High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 38.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. New Engl J Med. 2011;365:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 39.Raghu G, Scholand MB, de Andrade J, et al. Phase 2 Trial of FG-3019, Anti-CTGF Monoclonal Antibody in Idiopathic Pulmonary Fibrosis: Preliminary Safety and Efficacy Results. ATS-Congress, September 3rd. 2012 [Google Scholar]
- 40.Hoeper MM, Andreas S, Bastian A, et al. German Society of Cardiology (DGK), the German Society of Respiratory Medicine (DGP) and the German Society of Pediatric Cardiology (DGPK) Pulmonary hypertension due to chronic lung disease: updated recommendations of the Cologne Consensus Conference 2011. Int J Cardiol. 2011;152:45–53. [Google Scholar]
- E1.Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010;35:496–504. doi: 10.1183/09031936.00077309. [DOI] [PubMed] [Google Scholar]
- E2.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Seibold MA, Smith RW, Urbanek C, Groshong SD, Cosgrove GP, Brown KK, et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucociliary pseudostratified epithelium. PLoS ONE 8 (3) doi: 10.1371/journal.pone.0058658. e58658. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Bettencourt PE, Del Bono EA, Spiegelman D, Hertzmark E, Murphy RL. Clinical utility of chest auscultation in common pulmonary diseases. Am J Respir Crit Care Med. 1994;150:1291–1297. doi: 10.1164/ajrccm.150.5.7952555. [DOI] [PubMed] [Google Scholar]
- E5.Ghofrani HA, Wiedemann R, Rose F, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360:895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- E6.Madden BP, Allenby M, Loke T, et al. A potential role for sildenafil in the management of pulmonary hypertension in patients with parenchymal lung disease. Vascul Pharmacol. 2006;44:372–376. doi: 10.1016/j.vph.2006.01.013. [DOI] [PubMed] [Google Scholar]
- E7.Collard HR, Anstrom KJ, Schwarz MI, et al. Sildenafil improves walk distance in idiopathic pulmonary fibrosis. Chest. 2007;131:897–899. doi: 10.1378/chest.06-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E8.Han MK, Bach DS, Hagan PG, Yow E, Flaherty KR, Toews GB, Anstrom KJ, Martinez FJ. IPFnet Investigators Sildenafil preserves exercise capacity in patients with idiopathic pulmonary fibrosis and right-sided ventricular dysfunction. Chest. 2013;143:1699–1708. doi: 10.1378/chest.12-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Mermigkis C, Bouloukaki I, Antoniou KM, Mermigkis D, Psathakis K, Giannarakis I, et al. CPAP therapy in patients with idiopathic pulmonary fibrosis and obstructive sleep apnea: does it offer a better quality of life and sleep? Sleep Breath. 2013;17:1137–1143. doi: 10.1007/s11325-013-0813-8. [DOI] [PubMed] [Google Scholar]
- E10.Nathan SD, Basavaraj A, Reichner C, et al. Prevalence and impact of coronary artery disease in idiopathic pulmonary fibrosis. Respir Med. 2010;104:1035–1041. doi: 10.1016/j.rmed.2010.02.008. [DOI] [PubMed] [Google Scholar]
- E11.Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- E12.Lee JS, Ryu JH, Elicker BM, Lydell CP, Jones KD, Wolters PJ, et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:1390–1394. doi: 10.1164/rccm.201101-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Ryerson CJ, Arean PA, Berkeley J, Carrieri-Kohlman VL, Pantilat SZ, Landefeld CS, et al. Depression is a common and chronic comorbidity in patients with interstitial lung disease. Respirology. 2012;17:525–532. doi: 10.1111/j.1440-1843.2011.02122.x. [DOI] [PubMed] [Google Scholar]
- E14.Huppmann P, Sczepanski B, Boensch M, Winterkamp S, Schönheit-Kenn U, Neurohr C, et al. Effects of inpatient pulmonary rehabilitation in patients with interstitial lung disease. Eur Respir J. 2013;42:444–453. doi: 10.1183/09031936.00081512. [DOI] [PubMed] [Google Scholar]
- E15.Kenn K, Gloeckl R, Behr J. Pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis—a review. Respiration. 2013;86:89–99. doi: 10.1159/000354112. [DOI] [PubMed] [Google Scholar]