Abstract
Background
The incidence of invasive disease caused by encapsulated Haemophilus influenzae type f (Hif) has increased in the post-H. influenzae type b (Hib) vaccine era. We previously annotated the first complete Hif genome from a clinical isolate (KR494) that caused septic shock and necrotizing myositis. Here, the full genome of Hif KR494 was compared to sequenced reference strains Hib 10810, capsule type d (Hid) Rd Kw20, and finally nontypeable H. influenzae 3655. The goal was to identify possible genomic characteristics that may shed light upon the pathogenesis of Hif.
Results
The Hif KR494 genome exhibited large regions of synteny with other H. influenzae, but also distinct genome rearrangements. A predicted Hif core genome of 1390 genes was shared with the reference strains, and 6 unique genomic regions comprising half of the 191 unique coding sequences were revealed. The majority of these regions were inserted genetic fragments, most likely derived from the closely-related Haemophilus spp. including H. aegyptius, H. haemolyticus and H. parainfluenzae. Importantly, the KR494 genome possessed several putative virulence genes that were distinct from non-type f strains. These included the sap2 operon, aef3 fimbriae, and genes for kanamycin nucleotidyltranserase, iron-utilization proteins, and putative YadA-like trimeric autotransporters that may increase the bacterial virulence. Furthermore, Hif KR494 lacked a hisABCDEFGH operon for de novo histidine biosynthesis, hmg locus for lipooligosaccharide biosynthesis and biofilm formation, the Haemophilus antibiotic resistance island and a Haemophilus secondary molybdate transport system. We confirmed the histidine auxotrophy and kanamycin resistance in Hif by functional experiments. Moreover, the pattern of unique or missing genes of Hif KR494 was similar in 20 Hif clinical isolates obtained from different years and geographical areas. A cross-species comparison revealed that the Hif genome shared more characteristics with H. aegyptius than Hid and NTHi.
Conclusions
The genomic comparative analyses facilitated identification of genotypic characteristics that may be related to the specific virulence of Hif. In relation to non-type f H. influenzae strains, the Hif genome contains differences in components involved in metabolism and survival that may contribute to its invasiveness.
Electronic supplementary material
The online version of this article (doi:10.1186/1471-2164-15-38) contains supplementary material, which is available to authorized users.
Keywords: Comparative genomics, Haemophilus influenzae serotype f, Invasive, Pathogen, Synteny, Virulence factor
Background
Haemophilus influenzae is a Gram-negative coccobacillus that commonly dwells in the human upper respiratory tract. In addition to asymptomatic colonization, the species causes a wide spectrum of respiratory tract infections. H. influenzae is, for example, associated with acute otitis media in children, with sinusitis and pneumonia in adults as well as exacerbations in patients with chronic obstructive pulmonary disease (COPD). H. influenzae also occasionally causes invasive disease such as meningitis and septicemia [1, 2].
Isolates without a polysaccharide capsule are designated as nontypeable H. influenzae (NTHi), whereas encapsulated and hence typeable isolates are further divided into 6 serotypes designated a to f, depending on the capsular polysaccharide composition and antigenicity. NTHi is a commensal in the upper respiratory tract, and mainly causes upper respiratory tract infections. On the other hand, encapsulated strains, and most significantly capsule type b (Hib), are associated with systemic disease, and used to be a common cause of meningitis and epiglottitis in small children. However, the carriage and disease of Hib in developed countries has been greatly reduced since the 1990s due to the widespread use of Hib-specific vaccines [1]. Before introduction of the Hib vaccine, invasive non-Hib infections received little attention, being vastly outnumbered by severe Hib infections. However, epidemiological studies of invasive cases reported between 1989–2010 from North America and Europe indicate that invasive H. influenzae disease is now predominantly caused by NTHi and type f (Hif) [3–6]. Detailed analyses suggest that while NTHi acts as a true opportunist in systemic disease, Hif is also opportunistic (affecting frail individuals with underlying co-morbidities or predisposing conditions such as COPD, alcohol abuse, malignancy and diabetes), but often presents as a severe invasive disease in previously healthy and immunocompetent individuals [3, 7–10]. Importantly, more than half of the cases of invasive Hif infection presented in previously healthy individuals, and more than one third of these patients needed further treatment at intensive care units [3].
The genetic mechanism underlying the virulence of Hif is presently unknown, particularly when compared to Hib. Due to the increasing clinical significance of Hif, attempts to characterize established H. influenzae virulence factors including the capsule, lipooligosaccharide (LOS), hif fimbriae, the adhesin Hap as well as antibiotic and serum resistance have been performed in clinical Hif isolates [11–14]. With the objective to increase the current body of knowledge on Hif, we recently sequenced and annotated the complete genome of Hif KR494 [9, 15]. This clinical isolate caused necrotizing myositis and septic shock in a previously healthy 70-year old man. The Hif genome consists of 1856176 bp of chromosomal DNA, from which 1742 intact coding sequences (CDSs) were identified.
The primary objective of the present study was to use the de novo assembled genome to determine the genetic characteristics of Hif, focusing on differences compared with other sequenced H. influenzae genomes of varying serotypes and infection sites. The secondary objective was to study the genetic conservation of these features in different Hif clinical isolates, and possibly associate it with the phenotype. Our analyses enabled delineation of the accessory genome and revealed a plethora of Hif-specific genomic features including gene acquisition and gene loss that might play a critical role for virulence and host adaptation.
Methods
Bacterial strains
H. influenzae laboratory strains (n = 2) and clinical isolates (n = 21) used in the present study are listed in Table 1. Bacteria were grown on chocolate agar or in brain heart infusion (BHI) broth supplemented with NAD (Sigma-Aldrich, St. Louis, MO) and hemin (Merck, Darmstadt, Germany) (each at 10 μg/ml) at 37°C in a humid atmosphere containing 5% CO2.
Table 1.
Laboratory strains and clinical Haemophilus influenzae isolates used in the present study
| Strain | Serotype a | MLST b | Year of isolation | Isolation site c | Underlying conditions c | Clinical manifestation d | Gender | Age | Geographical location of isolation e | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Laboratory strains | ||||||||||
| MinnA | b | n/a | 1979 | CSF | n/a | Septic shock | n/a | Child | Minneapolis, USA | [16] |
| 3655 | Nontypeable | n/a | n/a | Middle ear | n/a | AOM | n/a | Child | Missouri, USA | [17] |
| Clinical isolates | ||||||||||
| KR494 | f | 124 | 2008 | Blood | Healthy | Septic shock | Male | 70 | Skåne | [9] |
| G19 | f | 124 | 2008 | Blood | n/a | n/a | Male | 57 | Gothenburg | [3] |
| G20 | f | 124 | 2008 | Blood | n/a | n/a | Male | 4 | Gothenburg | [3] |
| K238 | f | 124 | 2007 | Blood | n/a | n/a | Female | 63 | Stockholm | [3] |
| L11 | f | n/a | 2006 | Blood | MDS | Bacteremia/sepsis | Male | 65 | Skåne | [3] |
| L16 | f | n/a | 2007 | CSF | Healthy | Bacteremia/sepsis | Male | 74 | Skåne | [3] |
| L21 | f | 124 | 2008 | Blood | Healthy | Septic shock | Male | 82 | Skåne | [3] |
| L22 | f | 124 | 2008 | Blood | Severe COPD | Bacteremia/sepsis | Male | 81 | Skåne | [3] |
| L24 | f | 124 | 2009 | Blood | Healthy | Bacteremia/sepsis | Male | 65 | Skåne | [3] |
| L25 | f | 124 | 2009 | Blood | Healthy | Septic shock | Male | 51 | Skåne | [3] |
| L29 | f | 124 | 2009 | Blood | Healthy | Septic shock | Male | 77 | Skåne | [3] |
| L45 | f | n/a | 2005 | Blood | Healthy | Septic shock | Male | 51 | Skåne | [3] |
| L50 | f | 124 | 2007 | Blood | Healthy | Septic shock | Male | 63 | Skåne | [3] |
| L59 | f | n/a | 2007 | Blood | Healthy | Severe sepsis | Male | 71 | Skåne | [3] |
| M1 | f | 124 | 2009 | Blood | leucemia | Bacteremia/sepsis | Male | 3 | Skåne | [3] |
| M10 | f | 124 | 2008 | Blood | Solid tumor | Bacteremia/sepsis | Female | 77 | Skåne | [3] |
| M14 | f | 124 | 2008 | Blood | Solid tumor | Bacteremia/sepsis | Male | 63 | Skåne | [3] |
| M29 | f | n/a | 2006 | CSF | Healthy | Septic shock | Male | 0 | Skåne | [3] |
| M54 | f | n/a | 1999 | Blood | Healthy | Bacteremia/sepsis | Female | 78 | Skåne | [3] |
| S208 | f | 124 | 2008 | CSF | n/a | n/a | Female | 61 | Stockholm | [3] |
| S229 | f | 124 | 2008 | Blood | n/a | n/a | Female | 88 | Stockholm | [3] |
aIsolates were characterized by standard bacteriological techniques as described [3]. Strain serotype was verified by PCR whereas H. haemolyticus was excluded by 16S rRNA typing [3].
bMultilocus sequence typing (MLST) was performed as previously described [18].
cCOPD, chronic obstructive pulmonary disease; CSF, cerebrospinal fluid; MDS, myelodysplastic syndrome.
dAOM, acute otitis media.
eClinical isolates were from three different geographical areas of Sweden: Stockholm, Gothenburg and Skåne spanning from the years 1999–2009, isolated from blood or cerebrospinal fluid (CSF), but the clinical presentation, which was known for 15 of the 20 cases of invasive disease, ranged from Septic shock and epiglottitis to mild disease [3].
n/a, data not available.
Genome alignment and rearrangement
Full genome alignment and comparison of genomic rearrangement patterns between the Hif KR494 and reference strains (Table 2) was performed using the following programs: Mummer program [19], Artemis Comparison Tool (ACT) [20] or mVISTA [21] with BLASTn setting at a minimum identity of 95% and an expected threshold = 1e-5 unless otherwise indicated. For initial genome sequence pairwise alignment, a default setting value of 70% was used as the minimum percent conservation identity that must be maintained over the window size 11 for a region (> 50 bp) to be considered conserved. Thereafter, from the total identified conserved genomic blocks, a minimum of 95% of sequence identity was set to identify highly conserved regions (>50 bp). Genomic comparative maps were visualized using ACT, whereas Artemis [22] was used for data management. GenBank accession numbers of genomes used in the present study are listed in Table 2.
Table 2.
General genome features of Hif KR494 and the reference H. influenzae and Haemophilus spp. strains
| H. influenzae | H. aegyptius | H. haemolyticus | H. parainfluenzae | ||||
|---|---|---|---|---|---|---|---|
| Strain | KR494 | 10810 | RD Kw20 | 3655 | ATCC 11116 | M21639 | ATCC 33392 |
| Serotype | f | b | d | nontypeable | - | - | - |
| GenBank accession | CP005967 | NC_016809 | NC_000907 | AAZF01000000 | AFBC00000000 | AFQR01000000 | AEWU01000000 |
| Length (bp) | 1856176 | 1981535 | 1830138 | 1878368 | 1915025 | 2328161 | 2110314 |
| Whole genome GC content (%) | 38.05 | 38.14 | 38.15 | 38.02 | 38.08 | 38.59 | 39.18 |
| Genes GC content (%) | 38.68 | 38.76 | 38.83 | 38.75 | 39.00 | 39.33 | 39.83 |
| Coding content (%) | 88.54 | 87.29 | 84.66 | 89.38 | 90.07 | 74.29 | 91.59 |
| Genes avg. length (bp) | 913 | 910.79 | 935.59 | 870.79 | 856 | 887.6 | 962.55 |
| Total CDS number | 1742 | 1903 | 1657 | 1929 | 2020 | 2224 | 2010 |
| Strain-specific CDS a | - | 408 | 199 | 448 | 553 | 773 | 644 |
| Common CDS b | - | 1495 | 1458 | 1481 | 1487 | 1451 | 1366 |
| Unique CDS in Hif KR494 as compared to the other strains c | - | 247 | 284 | 261 | 255 | 292 | 376 |
aCDS number of each strain or species that was less conserved (protein sequence <85% similarity) or absent in the Hif KR494 genome.
bCDS number of each strain or species that was conserved and homologous to the Hif KR494 genome (protein sequence ≥85% similarity).
cNumber of CDS in Hif KR494 that were less conserved or absent as compared to the reference genomes (protein sequence <85% similarity).
Comparative analysis of gene content
To find unique and common genes in the Hif KR494 and reference strains/species, we performed extensive comparative analyses of open reading frames (ORFs) from whole genome sequences. We used the Mummer program in these analyses at window size 11. Briefly, total ORFs from KR494 and a selected reference genome, or of a reference genome pair, were analyzed with tBlastx at the setting of cutoff e-value ≤ 1e-5 and protein sequence similarity ≥85%. Finally, proteins with the best hits value from reciprocal blast were initially collected and grouped as (i) common CDSs shared between genomes, and (ii) CDSs unique to each genome. Results were formatted in Blast m8 tabular form. Thereafter, we used Perl scripts to further retrieve the accessory genome of Hif KR494 using different parameters, that is, genes absent from (i) all H. influenzae reference genomes used in the present study (Table 2), (ii) related Haemophilis spp. reference genomes (Table 2) or (iii) genes not found in all H. influenzae genome sequences available in the current databases. A similar approach was used to obtain common CDSs using the same parameters as outlined above. DNA plotter [23] and ACT were used for visualization of genomic features. In the present study, protein sequence homology (over the complete protein length) between Hif KR494 and reference strains was presented in percentage of similarity and identity.
Histidine auxotrophic assay
A histidine-dependent growth assay of H. influenzae isolates was performed with some modifications [24]. Two different media were used in this assay; Herriot defined media [25] with or without L-histidine denoted as w-His and w/o-His, respectively. Briefly, bacterial colonies were washed and resuspended in 5 ml of medium w/o-His to a cell density of OD600 0.5. Thereafter, 5 × 103 colony forming units (CFU) were separately inoculated into 10 ml of medium w/o-His and medium w-His supplemented with L-histidine at four different concentrations (0.0001%-0.01%). Here, the optimal concentration of L-histidine was empirically titrated for Hif isolates since the original Herriot defined medium was designed for H. influenzae serotype d [25]. Cultures were incubated for 12 h at 160 rpm and 37°C in a humid atmosphere containing 5% CO2. Bacterial growth was measured by spectrophotometry at OD600. BHI broth supplemented with NAD and hemin was used as a control medium.
PCR-based gene distribution studies
Genomic DNA was extracted with a GenElute Bacterial Genomic DNA Kit according to the manufacturer’s instruction (Sigma-Aldrich). Primers used in gene distribution studies are listed in supporting information (Additional file 1). Seventeen primer pairs (denoted as Hif_U1 to Hif_U17) were designed based upon the ORFs identified in the Hif KR494 genome sequence and used to screen unique genes/operons of the Hif accessory genome. The remaining primer pairs (Hif_M1 to Hif_M11) were used to study the missing genes and were designed based on the conserved ORFs from Hid Rd Kw20 unless otherwise indicated. PCR reactions were performed according to a standard protocol and conditions were as indicated in Additional file 1.
Antimicrobial susceptibility testing
Bacterial colonies were resuspended in sterile 0.9% NaCl to a density of McFarland standard 0.5. Antimicrobial susceptibility tests were performed using Etest® kanamycin strips (0.016-256 μg/ml) according to the manufacturer’s instruction (bioMérieux, Marcy l’Etoile, France). Minimum inhibitory concentration (MIC) of antibiotics was defined as the lowest concentration which fully inhibited the bacterial growth in comparison to antibiotic-free media.
Results
General features of the Hif KR494 genome
We first analyzed the KR494 genome based on a comparison with the well-established genomes of typeable and nontypeable H. influenzae that are available in the public databases. An overview of the complete genomes of Hif KR494 and selected reference strains are presented in Table 2. Genomes of NTHi are widely studied, and sequences from 18 distinct full NTHi genomes are available [26]. In contrast, only three annotated full genomes of typeable strains were available prior to this study, that is, Hib 10810, Hid Rd Kw20, and the recently reported Hif KR494 [15, 27, 28]. Due to their well-studied virulence characteristics and clinical significance, typeable strains of Hib 10810 and Hid Rd Kw20 in addition to NTHi 3655 were selected as references in our study. Regardless of the capsular serotype, the overall features of Hif KR494 were relatively similar to Hib 10810, Hid Rd Kw20 and NTHi 3655 (Table 2). The Hif KR494 genome was approximately 1.4% larger than the avirulent Rd Kw20, but 6.3% smaller as compared to Hib 10810. However, the Hif KR494 genome was smaller than the NTHi 3655 genome, but was relatively similar in size when compared to all NTHi genome sequences available (~1.8-1.95 Mb) [26, 29]. A similar G + C content (whole genome) of approximately ~38% was observed for the various serotypes (38.02-38.15%), whereas the average gene length varied only slightly between the different H. influenzae serotypes. In contrast, the numbers of predicted proteins and percentages of coding content in the four studied H. influenzae strains did not correlate with genome sizes and varied considerably. This could be due to the different annotation methods used for each genome. Notably, Rd Kw20 possessed the lowest number of CDSs, with the shortest genome among all analyzed.
A comparison of the genome organization between Hif KR494 and other H. influenzaestrains
We further analyzed the whole genome sequence similarity between Hif KR494 and the reference strains Hib 10810, Rd Kw20 and NTHi 3655. Due to genome rearrangement, whole genome DNA sequence similarity between strains appeared as a “block of conserved sequences” as analyzed by mVISTA. For this analysis, blocks were defined as contiguous regions of 50 bp to 100 kbp displaying a minimum of 70% nucleotide identity. These blocks were separated by genetic regions with lower levels of identity that could be of variable lengths in the aligned sequences. The Hif KR494 genome was aligned in 122 and 111 genomic blocks (comprising a sum of 774852 bp and 768482 bp, respectively, with at least 95% nucleotide identity) to the Hib 10810 and Hid Rd Kw20 genomes, respectively. The number of homologous blocks was reduced to 69 when Hif was aligned with the NTHi 3655 genome (a total of 494192 bp alignments with 95% identity). The data thus showed a high sequence similarity between the genome of Hif KR494 and the reference strains Hib 10810 and Hid Rd Kw20, whereas the Hif KR494 genome was slightly divergent from NTHi 3655.
An alignment using the ACT was done to identify inserted or deleted regions in the KR494 genome relative to the reference strains. Results showed large regions of synteny (genomic gene order) between Hif and these strains (Figure 1). Extensive synteny was observed between Hib 10810 and Hid Rd Kw20, which were distinct from Hif KR494 and NTHi 3655 (Additional file 2), indicating a closer relationship between Hib and Hid as compared with other types analyzed [18, 30]. Despite the gene organization and synteny of the Hif genome suggested a closer genetic relationship to the capsulated reference strains than to NTHi, numerous gene rearrangements were evident compared with all H. influenzae reference strains. Notably, multiple inversions were identified in the Hif KR494 genome, and were concentrated to three distinct regions; the 5′ end (within a ~450 kb fragment), the central region (within nucleotide positions 797–889 kb and 993–1254 kb), and finally at the 3′ end (~419 kb fragment).
Figure 1.
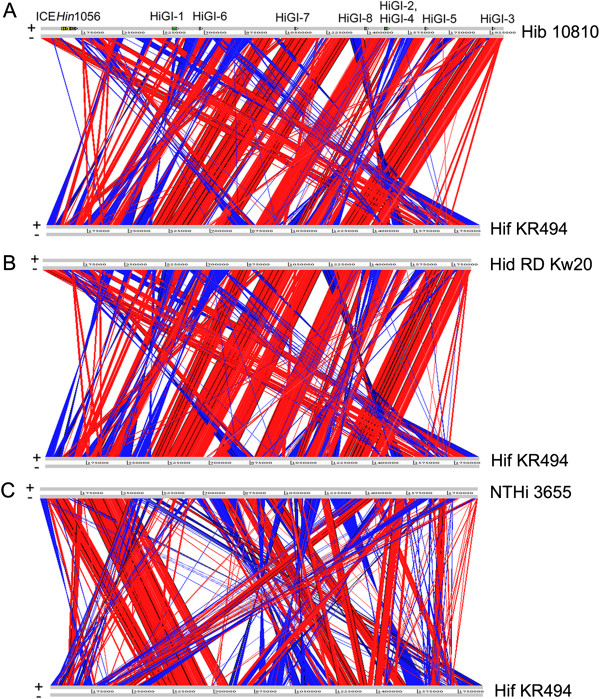
Genome comparison of H. influenzae type f KR494 and reference strains in ACT view. Genome alignment of Hif KR494 and (A) H. influenzae type b (Hib) 10810, (B) type d (Hid) Rd Kw20, and (C) nontypeable H. influenzae (NTHi) 3655. Respective genome designations are indicated on the right hand side of each genome line. Forward (+) and complement (−) strands of each genome are indicated in gray genome lines. Genomes are shown in full length and drawn to scale. Direct and inverted synteny between individual ORF (not indicated here) of the comparing genomes are shown in red and blue, respectively. The level of amino acid similarity is represented by colour shading with ascending saturation and indicates higher similarity. Genetic islands (HiGi) and the ICE element identified from Hib strain Eagan and strain 1056, respectively, are indicated for Hib 10810 in the upper genome line in panel (A).
In the Hif KR494 genome, 6 unique regions of difference (RgD) (i.e. relative to the reference strains) were identified and hereafter referred to as RgDF. The RgDFs were defined as regions in the Hif genome with a low level of conservation at the protein level relative to the reference strains (Hib 10810, Rd Kw20 and NTHi 3655), and containing a minimum of 5 neighboring CDSs with <85% sequence similarity. The RgDFs comprised a total of 144893 bp or 7.8% of the full genome (Figure 2). With the exception of RgDF3 and 6, the G + C contents of the remaining RgDFs (range 35.03-39.28%) were distinct from the remaining part of the genome (38.05%) (Additional file 3). This clearly indicated that the RgDFs were acquired from foreign source(s). The RgDFs contained several traits common to mobile genetic elements, including tRNA and rRNA genes, integrases (catalyze unidirectional DNA recombination), and transposases (catalyze movement of transposons). The majority of phage-related genes were found at RgDF1, 3 and 4, which were considered as prophage islands. The RgDF1, 4, 5 and 6 included the predicted Hif KR494 genomic islands (GifKR494) 11, 13, 16 and 21, respectively (Additional file 4).
Figure 2.
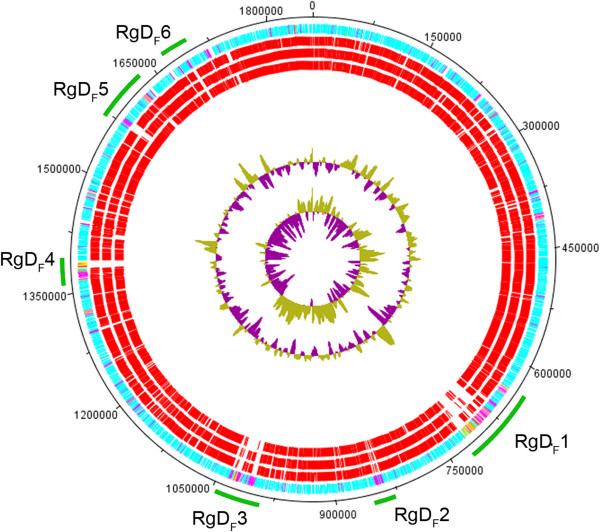
Map of region of differences (RgD F ) of the H. influenzae type f KR494 genome. Circular representation of protein conservation between Hif KR494 and reference strains was visualized using DNA plotter. From the outside in, the outer circle shows the genome length of Hif KR494 with position markers. The second circle shows the total ORFs of KR494 genome predicted on both forward and reverse strands. Common and unique ORFs relative to the reference strains are colored in blue and magenta, respectively. Phage-related ORFs are marked in yellow and orange. The third to fifth circles represent the distribution of individual ORF with high homology (≥85% similarity) (in red) to the corresponding ORF of reference strains, Hib 10810, Hid Rd Kw20 and NTHi 3655, respectively. Gaps between the conserved ORFs represent region of difference in the Hif KR494 genome, and were denoted as RgDF1 to 6 (marked with green lines). The GC plot and GC skew of the Hif KR494 genome are shown in the sixth and seventh circle, respectively.
The Hif KR494 accessory genome
A pairwise BlastP comparison revealed 1390 CDSs of Hif KR494 shared with Hib 10810, Hid Rd Kw20 and NTHi 3655 when 85% similarity was used as cutoff [31, 32] (Figure 3A). The conserved CDSs included the H. influenzae-specific genes that were previously delineated as the H. influenzae core-genome (i.e. found in every strain) [26]. The distribution of gene functionality was analyzed based upon the Cluster of Orthologous Groups (COGs) protein database [33]. Besides the CDSs of unknown function, the majority of core genes were involved in protein translation, amino acid metabolism and cell wall biogenesis (Figure 3B). Since the H. influenzae core genome has been widely studied [26, 31, 34–37], the Hif counterpart was not further analyzed in the present study. Our analyses also showed that 408 CDSs were unique to Hib 10810, 199 CDSs were unique to Hid Rd Kw20, and finally 448 CDSs were unique to NTHi 3655 when compared to the Hif KR494 genome (Figure 3A).
Figure 3.
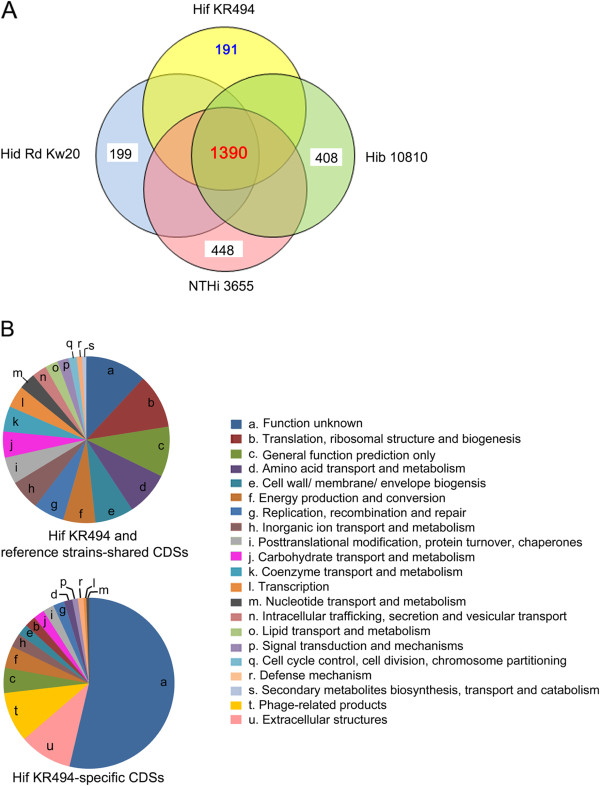
Comparative genomic overview of H. influenzae type f KR494 and reference strains. (A) A VENN diagrame depicts the number of commonly shared and strain-specific CDSs by Hif KR494 and reference strains. The total number of CDSs that are specific to Hif KR494 and conserved in all strains are shown in blue and red fonts, respectively. The number of strain-specific CDSs in Hib 10810, Hid RD Kw20 and NTHi 3655 compared to Hif KR494 are shown in black font. (B) Functional classification of subsets of KR494 CDSs shown in Panel (A). Delineation was based on the COG database.
Although Hif and the reference strains shared many homologous proteins, a detailed comparison revealed that 11% (191 CDSs) of the total annotated genes in the Hif KR494 genome were less conserved or absent from the reference genomes. These genes were thus further referred to as the Hif unique CDSs or accessory genes (Figure 3A, Table 3), which means that they were found in Hif KR494 only, but absent in the reference strains Hib10810, RdKw20 and NTHi3655. The distribution of accessory genes in the Hif KR494 genome verified the findings obtained in studies on the H. influenzae supragenome. Two previous supragenome studies revealed that 10-19% of the gene content in any H. influenzae genome is generally related to strain-specific accessory genes [26, 35]. As shown in Figure 3B, with the exception of products of unknown function, a significant number of the unique CDSs were associated with extracellular structures, i.e., fimbriae and trimeric autotransporters. We also identified a number of unique CDSs encoding phage-related products. However, among all unique CDSs of Hif KR494, the majority (65.5%) showed a low homology or no significant hits in the H. influenzae genomes (summarized in Table 3).
Table 3.
Unique genes in Hif KR494 in relative to Hib 10810, Rd Kw20 and NTHi 3655
| Locus a | Function | G + C b (%) | Homologue species | Similarity/Identity c (%) | USS d | Mobile element e | RgD F /Gif KR494 |
|---|---|---|---|---|---|---|---|
| Capsule | |||||||
| HifGL_000665- HifGL_000673 | Serotype-f capsule biosynthesis locus | 25.5 | H. influenzae 700222 (type f) | 100/99.0-100 | - | - | RgDF1 |
| Iron transport and metabolism | |||||||
| HifGL_000676- HifGL_000685* | sap2ABCDF | 38.3 | H. parainfluenzae ATCC 33392 | 94.0-100/87.0-96.0 | 4 | Two class-LINE transposons (overlap with sap2C and transposase HifGL_000684); two transposase (HifGL_000683, HifGL_000684); phage island at downstream | RgDF1 |
| HifGL_001444* | Iron Fe3+ ABC superfamily ATP binding cassette transporter | 39.7 | H. haemolyticus M21639 | 98.0/98.0 | 3 | A class LINE/CR1 Transposon | - |
| HifGL_001664* | Heme-binding protein HutZ | 38.5 | P. multocida HN06 | 98.0/99.0 | - | - | - |
| Motility | |||||||
| HifGL_000989- HifGL_000994* | Fimbriae aef3abcdef | 39.5 | H. aegyptius ATCC 11116 | 62.0/52.0 (aef3a), 89.0-99.0/84.0-99.0 (aef3bcdef) | 4 | Flanked by two class LTR-Gypsy transposon (overlaps HifGL_000986) and transposase (HifGL_000996); phage island at downstream | RgDF3 |
| Energy production and conversion | |||||||
| HifGL_001349- HifGL_001358* | Rnf electron transport complex (sohB-rnfABD) | 39.8 | H. haemolyticus M19501 | 91.0-100/85.0-100.0 | 12 | Two identical direct repeats (41 bp) at HifGL_001351 and HifGL_001357; phage island at downstream | RgDF4 |
| Glycerol transport and metabolism of glycerophospholipid f | |||||||
| HifGL_000396- HifGL_000397 (subunit gene duplication in glpKFQT operon) | Glycerol kinase GlpK | 41.6 | H. influenzae PittGGf | 99.0/99.0 | - | - | - |
| Glycerol facilitator protein GlpF | 43.6 | H. influenzae PittGGf | 99.0/99.0 | - | - | - | |
| Posttranslational modification, protein turnover, chaperones f | |||||||
| HifGL_000630-HifGL_000632 (duplication of three-gene cluster) | Phosphoethanolamine transferase | 31.6 | H. influenzae NT127f | 99.0/99.0 | - | - | - |
| tRNA modification GTPase TrmE | 39.7 | H. influenzae NT127f | 99.0/97.0 | - | - | - | |
| Peptidylprolyl isomerase | 34.3 | H. influenzae R3021f | 99.0/99.0 | - | - | - | |
| Sugar and amino acid transport and metabolism f | |||||||
| HifGL_000838- HifGL_000849 (a duplication of five-genes cluster) | Methyltransferase | 37.4 | H. influenzae R2866f | 100/99.0 | 8 | - | RgDF2 |
| Glycerol-3-phosphate regulon repressor GlpR | 29.3 | Mannheimia succiniciproducens MBEL55E | 83.0/60.0 | ||||
| Glycerol-3-phosphate regulon repressor GlpR | 33.2 | Aggregatibacter actinomycetemcomitans D11S-1 | 93.0/85.0 | ||||
| Putative carbohydrate diacid regulator | 37.1 | H. influenzae R3021f | 99.0/99.0 | ||||
| Gluconate proton symporter | 44.1 | H. influenzae NT127f | 100/100 | ||||
| Kanamycin resistance (KNTase protein) | |||||||
| HifGL_000799* | DNA polymerase beta domain protein region | 32.4 | M. haemolytica PHL213 | 77.0/63.0 | - | - | - |
| HifGL_000800* | Nucleotidyltransferase substrate binding protein | 30.7 | M. haemolytica PHL213 | 81.0/64.0 | - | - | - |
| Replication, recombination and repair | |||||||
| HifGL000956 | DNA-damage-inducible protein J, negative regulator of translation | 35.1 | H. influenzae biogroup aegytptius F3031 (BPF) | 98.0/97.0 | - | - | - |
| HifGL001635* | Type II restriction enzyme HinfI | 27.4 | H. influenzae Rf (type f) | 100/100 | - | - | RgDF6 |
| HifGL001636* | Adenine-specific methyltransferase HinfI | 32.5 | H. influenzae Rf (type f) | 100/100 | - | - | RgDF6 |
| Unknown function hydrolase | |||||||
| HifGL_000176*; | Cell wall-associated hydrolase | ~48.0-49.0 | H. haemolyticus M21639 | 100/100 | - | Three transposons LINE/R2, LINE/R1 and LTR/Copia | GifKR494-2 |
| HifGL_000233; | GifKR494-8 | ||||||
| HifGL_001417; | GifKR494-14 | ||||||
| HifGL_001593; | GifKR494-17 | ||||||
| HifGL_001616; | GifKR494-19 | ||||||
| HifGL_001665 | GifKR494-22 | ||||||
| Trimeric autotransporter of unknown function | |||||||
| HifGL_000054 | Putative YadA-like protein | 43.3 | H. haemolyticus M19107 | 83.0/ 77.0 | - | - | - |
| HifGL_000835 | Hep_Hag family protein | 43.5 | Neisseria mucosa ATCC 25996 | 63.0/ 45.0 | - | - | - |
| HifGL_000837* | Putative target SNARE coiled-coil domain-containing protein | 37.9 | H. haemolyticus M19501 | 83.0/ 75.0 | 1 | - | - |
| HifGL_000844 | Putative YadA-like protein | 40.3 | H. haemolyticus M19501 | 99.0/ 99.0 | 1 | - | - |
| HifGL_001158 | Hep_Hag superfamily protein | 32.9 | H. aegyptius ATCC 11116 | 97.0/ 85.0 | - | - | - |
| HifGL_001217 | Trimeric autotransporter adhesin | 37.4 | H. influenzae biogroup aegytptius F3031 (BPF) | 86.0/ 83.0 | - | - | - |
| HifGL_001431* | Trimeric autotransporter adhesin | 40.7 | H. influenzae biogroup aegytptius F3047 | 86.0/ 80.0 | 1 | - | - |
| HifGL_001626 | YadA/hemagluttinin like protein | 40.8 | H. aegyptius ATCC 11116 | 88.0/84.0 | - | - | RgDF6 |
| Other metabolic genes | |||||||
| HifGL000310* | Glutathione S-transferase domain-containing protein | 40.8 | Actinobacillus ureae ATCC 25976 | 98.0/99.0 | - | - | - |
| HifGL000957 | Addiction module antitoxin/rele toxin-like protein, plasmid stabilization system | 33.3 | H. haemolyticus M21127 | 100/98.0 | - | - | - |
| HifGL001004 | Phospholipase/carboxylesterase | 33.1 | A. succinogenes 130Z | 89.0/79.0 | - | - | - |
| HifGL001672* | Putative lipoprotein | 33.8 | H. haemolyticus M19501 | 95.0/93.0 | - | - | - |
aUnique CDS of hypothetical proteins and phage products are not included.
bAverage G + C content is presented for single locus or as an average value of the indicated loci cluster or operon.
cProtein sequence similarity and identity (over the complete protein length) is presented in percentage as individual value for single CDS or as range of value for clustered CDSs.
d Haemophilus influenzae uptake signal sequence (USS) (AAGTGCGGT).
eMobile elements include phage island, transposase, tandem repeats and transposons. Transposon sequences and transposase, respectively, were predicted based on transposon database using softwares of RepeatMasker and RepeatProteinMask (http://www.repeatmasker.org/). Tandem repeat sequences were predicted by using Tandem Repeat Finder (http://tandem.bu.edu/trf/trf.html). Prophage islands were predicted using Prohinder software and ACLAME database (http://aclame.ulb.ac.be/), thereafter we performed BLAST comparison (standalone BLAT v.34) between KR494 genome and prophage database.
fThese genes that exist in the Hif KR494 genome as a two-copy number resulted in unusual allelic duplication in the Hif KR494 genome. Despite these genes were considered unique in KR494 when compared to Hib 10810, Rd Kw20 and NTHi 3655, they also shared homology with NTHi PittGG, NT127, R3021 and R3866 that were not included as reference strains in the present study.
*Target single locus or contiguous loci assessed in gene distribution studies (Table 5).
Putative virulence and metabolic genes unique to H. influenzaetype f KR494
Approximately half of the total unique genes in Hif KR494 (114 CDSs) were located within the RgDFs. Notably, some of these unique CDSs resulted in duplication of paralogous genes (homologous genes present in the same strain, in this case Hif KR494) involved in virulence and iron utilization. In addition, allelic duplication was identified for genes involved in tRNA modification as well as in transport and metabolism of amino acids, sugar and glycerol (Table 3).
The “sensitivity to antimicrobial peptide” (Sap) transporter of H. influenzae is a six-subunit multifunctional inner membrane ABC transport protein complex important for resistance against antimicrobial peptides (AMPs) [38, 39]. It consists of a periplasmic solute binding protein (subunit SapA), transmembrane permeases complex (SapB and SapC), ATPase subunits (SapD and SapF) and a subunit SapZ of unknown function. Two sap operons were identified in the Hif KR494 genome, a highly conserved H. influenzae sapABCDFZ operon (HifGL_001309-HifGL_001314), and an additional five-gene sap operon encoded by unique Hif CDSs (HifGL_000676-HifGL_000685). The additional Sap operon shared a high protein sequence homology (94-100% similarity) with the SapABCDF operon of H. parainfluenzae ATCC33392 (corresponding locus in ATCC33392: HMPREF9417_1073 to HMPREF9417_1077), which is distinct from the conventional sap of H. influenzae (Table 3). Moreover, the Hif KR494 additional sap operon lacked sapZ and its 5′ end coded for a COG3106 family hypothetical protein (HifGL_000675), and the entire gene organization was analogous to the H. parainfluezae sap operon (Figure 4A). We thus annotated the unique operon in Hif KR494 as sap2ABCDF, and the numerical designation was to distinguish it from the “conventional” sap operon.
Figure 4.
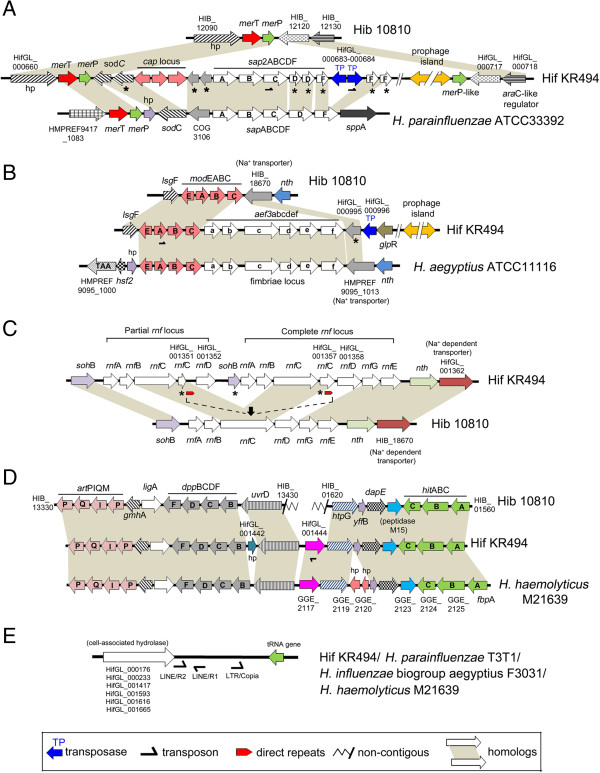
Genomic structures and organizations of unique genes in H. influenzae type f KR494. Organization of loci of specific genes in Hif KR494 were compared with reference strains or closely-related species. Genomes of respective reference species or strains are indicated on the right hand side of each panel. The flanking genes and genomic organization of (A) sap2ABCDF, (B) fimbriae gene cluster aef3abcdef, (C) duplet rnf electron transport complex, (D) unique iron-binding transporter HifGL_001444, and (E) genetic island structure of cell wall-associated hydrolase of KR494, were analogous to the indicated reference species while the unique genes were absent from Hib 10810 (a representative of H. influenzae reference genomes). Asterisks indicate partial CDSs. Hypothetical proteins of unknown function are denoted as “hp”. Homologous genes are indicated with gray shading. In panel (A), the predicted protein products of sap2D and sap2F (ATPase subunits) are shorter than their counterparts in H. parainfluenzae. The loss of a functional ATPase complex (sap2DF) might be compensated by the subunit product (SapD and SapF) from the H. influenzae conserved Sap system. In panel (C), two identical 41 bp direct repeats were identified at 40 bp upstream of HifGL_001352 (rnfD) and at the first 220 bp of HifGL_001357 (rnfC), respectively. The repeats may mark the two edges of the inserted genomic fragment suggesting the intergenic region between HifGL_001351 and HifGL_001358 as the insertion site. The black arrow indicates the possible insertion at the original rnfC subunit gene. The insertion may also have resulted in partial CDSs of the neighboring loci, HifGL_001351 and HifGL_001357. Both loci encode rnfC but with internal stop codon thus may not encode a functional protein. Nevertheless, the functionality of the rnf operon might not be affected since the intact CDS of rnfC were retained at HifGL_001350 and HifGL_001356.
Interestingly, the well-studied Hif cap locus (HifGL_000665-HifGL_000673) was located a few CDSs upstream of the sap2 operon. The Hif KR494 cap locus was organized in a sodC-cap arrangement, a typical gene organization of group II capsule biosynthesis loci [13, 40]. A prophage island (HifGL_000691-HifGL000715) was located downstream of the sap2, and this island contained high numbers (15 CDSs) of H. influenzae and H. aegyptius phage protein homologues that were interspersed by hypothetical proteins (10 CDSs). The Hif capsule locus, sap2 and the prophage island together formed the RgDF1. The sap2 operon carried two class-LINE transposons (one overlapped with sap2C and one with transposase HifGL_000684) and two transposase genes (HifGL_000683 and HifGL_000684) that resembled a composite transposon-like structure (Figure 4A).
Fimbriae, also designated as hemagglutinin pili, are crucial for H. influenzae adherence and colonization in the upper respiratory tract [41, 42]. In addition to the classical Haemophilus fimbriae locus hifACDE of genotype IIIb (HifGL_001282-HifGL_001285) that does not encode the periplasmic chaperon of subunit HifB [43], the Hif KR494 genome possessed another six-gene fimbriae cluster (HifGL_000989-HifGL_000994). The second fimbriae locus in Hif KR494 was found in the RgDF3, and had a high similarity (89-99% except for aefA = 62%) with the Aef fimbriae of H. aegyptius ATCC11116 (corresponding locus HMPREF9095_1007-HMPREF9095_1012) (Table 3). The aef homologue was not found in other H. influenzae, but is present as an aef3abcdef cluster in the conjunctivitis strain H. influenzae biogroup aegyptius F3047 (corresponding locus HICON_14070-HICON_14120) [29, 44]. We annotated the second Hif KR494 fimbriae cluster as aef3abcdef. While the hif cluster was inserted at the conserved region between purE and pepN in the Hif KR494 chromosome, the aef3 cluster was located at a unique position between the modC gene (HifGL_000988) and the sodium dependent transporter gene (HifGL_000995), analogous to the gene order found in H. aegyptius and H. influenzae biogroup aegyptius F3047 (Figure 4B). Two flanking mobile genetic elements were identified; a class LTR-Gypsy transposon (overlapped locus modA; HifGL_000986) located upstream of the aef3 cluster, and a transposase IS1016C2 (HifGL_000996) located downstream of the same cluster, suggesting a composite-like structure of aef3. In addition, a small prophage island (HifGL_1021-HifGL_1026) was found a few CDSs downstream of the aef3 cluster.
NADH oxidoreductase is a six-subunit enzyme complex responsible for electron transfer to nitrogenase during nitrogen fixation [45]. The enzyme complex is encoded by a single copy rnfABCDGE operon that is highly conserved in H. influenzae. However, the Hif KR494 genome had two contiguous rnf operons (HifGL_001348-HifGL_001360) consisting of a partial (rnfABCD) and a complete operon (rnfABCDGE) (Figure 4C). This may have been caused by insertion of an additional rnf gene cluster (HifGL_001352-HifGL_001357) via homologous integration between subunit genes of the original rnf operon. The suggested mechanism of gene insertion is illustrated in Figure 4C. Interestingly, the protein products of rnfA, rnfB and rnfD in Hif KR494 were homologous (98-100% similarity) to the corresponding Rnf proteins of H. haemolyticus (Table 3). Moreover, the third prophage island (HifGL_001362-HifGL_001379) that mainly encodes H. haemolyticus phage proteins was located downstream the Hif KR494 rnf operon. Both the rnf gene cluster and the adjacent prophage island were located on RgDF4.
RgDF6 contained unique CDS for the type-II restriction enzyme HinfI (HifGL_001635) and modification methylase HinfI (HifGL_001636) that were previously described in H. influenzae Rf (serotype f) [46]. The gene products are important for Hif to survive infection by a variety of phages. These genes have neither been identified in any non-type f H. influenzae genome nor characterized in previous genotyping studies of multiple Hif isolates [11, 12].
There were also a number of unique Hif CDSs that were not associated with the RgDFs (Table 3). These included two unique Hif CDSs (HifGL_000799 and HifGL_000800) that encoded hypothetical proteins containing nucleotidyltransferase and a substrate-binding domain (pfam08780 homologues of Staphylococcus aureus kanamycin nucleotidyltranserase (KNTase)), respectively. In S. aureus, the KNTase is a plasmid-encoded enzyme that confers resistance to a wide range of aminoglycoside antibiotics including kanamycin A [47, 48]. A BLAST analysis revealed that HifGL_000799 and HifGL_000800 are not present in other H. influenzae but have moderate homology with the hypothetical proteins MHA_2776 (77% similarity) and MHA_2775 (81% similarity) of Mannheimia haemolytica PHL213, respectively, and also share the direct synteny of flanking genes (illustration not shown).
The Hif KR494 genome also harbored two genes involved in heme/iron utilization (HifGL_001444 and HifGL_001664) that have not been previously found in H. influenzae. The HifGL_001444 encodes a product of high homology (98% similarity) to the iron (Fe3+) ABC transporter substrate-binding protein of H. haemolyticus M21639 (locus in M21639: GGE_2117) (Table 3). It is distinct from the previously described heme/iron uptake systems in H. influenzae, i.e., HxuABC, DppBCDF, hFbpABC, HbpA, Hgp, Hup, TbpAB, HipABC, P4 and Sap [38, 49–51]. Moreover, 11 upstream and 7 downstream flanking genes of HifGL_001444 had a similar gene order as in the H. haemolyticus M21639 genome (Figure 4D). The gene order included two relevant yet highly conserved H. influenzae heme/iron uptake systems, the dppBCDF gene cluster (HifGL_001438-HifGL_001441) and the hitABC operon (hFbpABC complex) (HifGL_001449-HifGL_001451). However, these were in different gene order as compared to the known H. influenzae reference genomes (Figure 4D). The HifGL_001664 encodes a heme-binding HutZ homologue (98% similarity) (GenBank number: YP_005362747) of Pasteurella multocida[52]. However, two typical gene partners of hutZ in a triplet gene operon, hutX and hutW of unknown function, were not present.
Another unique feature of the Hif KR494 genome was the presence of 6 copies of a CDS located on six ~3.0 kbp long genetic islands (GifKR494-2, 8, 14, 17, 19 and 22) (Figure 4E, Table 3, Additional file 4). The CDS encodes a protein with high homology (100% similarity) to the cell-wall associated hydrolase (YP_004135972) of Brazilian purpuric fever (BPF) clone F3031 of H. influenzae biogroup aegyptius, H. haemolyticus M21639 (EGT78466) and H. parainfluenzae T3T1 (YP_004822531). No homologue of this protein has previously been found in other H. influenzae. Three transposons of class LINE/R2, LINE/R1 and LTR/Copia were consistently found at 41 bp, 336 bp and 1045 bp downstream of these CDSs, respectively. A tRNA gene was also present at ~2.5 kbp downstream of the CDSs. This implied that the cell-wall associated hydrolase has been horizontally acquired as a genetic island and subsequently integrated at the adjacent tRNA gene. Finally, additional genes encoding for a total of 7 unique YadA-like trimeric autotransporters with varying lengths (213-629aa) were also identified (Table 3). The exact function of these proteins is presently unknown.
Gene deletions in the Hif KR494 genome
The Hif KR494 genome was also compared to the reference strains in order to define absent genes, that is, missing gene or gene loss in Hif KR494 but present in the reference strains Hib 10810, Rd Kw20 and NTHi 3655. Notably, when compared to Hib 10810, neither the previously described Hib genetic islands (HiGI-1 to HiGI-8 except for HiGI-6; originally identified from strain Hib Eagan) nor the integrative and conjugative element (ICE) Hin1056 (strain Hib 1056) were present in the Hif KR494 genome (Figure 1A) [53–56]. The ICEHin1056 is known to confer ampicillin, tetracycline and chloramphenicol resistance among H. influenzae transconjugants [54].
The Hif KR494 missing genes of non-hypothetical and non-phage proteins are summarized in Table 4. Unlike the majority of H. influenzae strains, the Hif KR494 genome did not retain the hisABCDEFGH operon (corresponding locus in Rd Kw20: HI0468-HI0474) and hisIE (HI0475). The operon encodes 8 enzymes that co-operatively catalyze the formation of L-histidine from phosphorybosyl phyrophosphate, a crucial pathway in histidine biosynthesis [57]. Another important operon that was absent in the Hif KR494 was the eight-gene hmg locus (HI0867-HI0874) involved in LOS biosynthesis. The hmg locus is responsible for incorporation of sialyl- and (PEtn → 6)-α-D-GalρNAc containing tetrasaccharide units which results in high molecular weight-glycoforms of LOS [58, 59].
Table 4.
Genes of reference H. influenzae strains that are absent in the Hif KR494 genome
| Locus a,b | Gene/function |
|---|---|
| Histidine biosynthesis pathway | |
| HI0468-HI0474* | hisABCDEFGH operon |
| HI0475 | hisIE |
| Other amino acid biosynthesis pathway | |
| HI0607 | Shikimate 5-dehydrogenase, aroE (phenylalanine, tyrosine and tryptophan) |
| HI0737 | Acetohydroxy acid synthase II (valine, leucine and isoleucine) |
| Anaerobic fermentation of L-ascorbate | |
| HI1024-HI1026* | ulaDEF operon |
| HI1027 | Lyx |
| HI1031 | 2,3-diketo-L-gulonate reductase |
| Folate biosynthesis | |
| HI1190* | 6-pyruvoyl tetrahydrobiopterin synthase |
| HI1464 | Dihydropteroate synthase |
| Secondary molybdenum transporter | |
| HI1469 | modA |
| HI1470-HI1472* | molABC |
| HI1473-HI1475 | modD-salX-cysU |
| HI1525 | modA |
| LOS biosynthesis | |
| HI0867-HI0874* | hmg locus (siaA, wbaP, rffG) |
| HI1578 | Glycosyl transferase WcaA |
| HIB_08850c | UDP-glucose--lipooligosaccharide glucosyltransferase |
| Iron and zinc transporter | |
| HIB_07090c, * | Zinc transporter ZitB |
| HI0291 | Periplasmic mercury transport-like protein |
| HI0661 | Hemoglobin-binding protein OapA |
| HI0712 | Hemoglobin-binding protein |
| Other metabolism | |
| HI0053 | Zinc-type alcohol dehydrogenase (fructose and mannose metabolism) |
| HI0692 | Xanthine-guanine phosphoribosyltransferase (purine metabolism) |
| HI1649 | D-lactate dehydrogenase (pyruvate metabolism) |
| Others | |
| HI0584 | Peptidase, aminobenzoyl-glutamate utilization protein |
| HI0947 | Virulence-associated protein C |
| HI1251 | Virulence-associated protein A |
| HI1465 | Cell division FtsH-like protein |
aCDSs of hypothetical proteins and phage products are not included.
bLocus tags are based on the genome of Rd Kw20 unless otherwise is indicated.
cLocus tags are based on the genome of Hib 10810.
*Target single locus or contiguous loci assessed gene distribution studies (Table 6).
Multiple molybdate transport systems of different affinities have previously been described in H. influenzae Rd Kw20 [60, 61]. In contrast to the high affinity Haemophilus primary molybdenum transporter operon modABCE that remained intact (HifGL_000985-HifGL_000988), the entire molABC-modAD-salX gene cluster (HI1469-HI1475) that encodes the secondary molybdenum transporter system was missing in the Hif KR494 genome. The gene for a ZitB zinc transporter (corresponding locus in Hib 10810: HIB_07090) was also absent in Hif KR494 genome. In parallel, the genes HI1024-HI1027 and HI1031 coding for enzymes involved in anaerobic fermentation of L-ascorbate as an alternative carbon source were not present in the Hif KR494 genome. Consistent with the previous description of Hif isolates [11, 12], the Hif KR494 genome did not harbor genes for the high molecular weight adhesin (hmwAB), which is a common virulence factor in NTHi.
The unique genetic properties of Hif KR494 are conserved in clinical type f isolates
To investigate whether the distinct genomic features in the Hif KR494 genome were conserved in the serotype f lineage, we investigated the distribution of the unique and missing genes in 20 clinical Hif isolates using a PCR-based screening. The clinical isolates were chosen from different years and from various geographical areas of Sweden (Table 1). The severity of clinical disease had been established for most isolates used in the present study, and ranged from mild disease in immunocompromised individuals to septic shock in previously healthy subjects.
To avoid orthologs, (homologous genes present in heterologous H. influenzae strains), we targeted 17 unique CDSs that lack homology with the H. influenzae accessory gene pool available in the database (Table 5, Additional file 1). The well-studied NTHi 3655 and Hib MinnA (genetically clonal to Hib 10810) were used as negative controls, whereas Hif KR494 represented the positive control. Using primers based upon Hif KR494 sequences, 10 Hif isolates were positive for all the unique CDSs screened, indicating the presence of the targeted genes, whereas the remaining isolates were negative for one to a maximum of four genes (Table 5).
Table 5.
Distribution of Hif KR494 unique genes in clinical Hif isolates
| Locus tag a (HifGL) | Description b | H. influenzae serotype f clinical isolates | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KR494 | G19 | G20 | K238 | L11 | L16 | L21 | L22 | L24 | L25 | L29 | L45 | L50 | L59 | M1 | M10 | M14 | M29 | M54 | S208 | S229 | No. of isolate c | Hib MinnA | NTHi 3655 | ||
| 000176 | cw-hydrolase | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | 19 | +d | +d |
| 000310 | GST | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 20 | - | - |
| 000674a | RgDF1: sap2 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | + | 19 | - | - |
| 000799a | KNTase | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 20 | - | - |
| 000834a | RgDF 2: glpR | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | 19 | - | - |
| 000837 | TAA | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 20 | - | - |
| 000989a | RgDF 3: aef3 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 20 | - | - |
| 001007a | RgDF 3:PI | + | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | - | + | + | 18 | - | - |
| 001350a | RgDF 4: rnf | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | - | + | + | + | + | + | 18 | - | - |
| 001363a | RgDF 4: PI | + | + | + | + | + | + | + | + | + | + | + | + | + | +e | - | + | + | + | + | + | + | 19 | - | - |
| 001431 | TAA | + | - | - | + | + | - | + | + | - | + | + | - | + | - | + | - | - | + | + | + | + | 12 | - | - |
| 001444 | Fe3+ transporter | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 20 | - | - |
| 001463 | UHP | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 20 | - | - |
| 001635 | RgDF 6: RHinfI | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 19 | - | - |
| 001636 | RgDF 6: MHinfI | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | 19 | - | - |
| 001664 | HutZ | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 20 | - | - |
| 001672 | Lpp | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 20 | - | - |
| No. of CDS present | 17 | 16 | 15 | 17 | 17 | 16 | 17 | 17 | 16 | 17 | 17 | 16 | 17 | 13 | 14 | 14 | 16 | 17 | 16 | 17 | 17 | 1 | 1 | ||
aLocus tags were based on the Hif KR494 genome [15]. For PCRs targeting the contiguous loci, only the first locus is shown. Full information of the target genes, amplicon size and PCR conditions is described in Additional file 1.
bAbbreviations: cw-hydrolase, cell wall-associated hydrolase; glpR, duplication of gene cluster involved in sugar and amino acid transport and metabolism; GST, glutathione S-transferase domain-containing protein; KNTase, kanamycin nucleotidyltranserase; Lpp,unknown lipoprotein; MHinfI,type f-specific adenine-specific methyltransferase HinfI; PI, prophage island; TAA, trimeric autotransporter; UHP, unknown function hypothetical protein; RHinfI,Type II restriction enzyme HinfI.
cTotal number of Hif clinical isolates (excluding Hif KR494) with unique genes found in Hif KR494.
dCDS was not found despite the presence of primer priming site in the NTHi 3655 genome (AAZF01000000) and Hib 10810 (NC_016809); PCR product had a smaller size.
ePositive, but with a larger PCR product.
+PCR positive (CDS present); -PCR negative (CDS absent).
In the screening of gene loss in Hif (in total 11 CDSs) (Table 6), the NTHi 3655 and Hib MinnA were used as positive controls. The LOS hmg locus (siaA and wbaP) was lacking in all isolates analyzed, whereas 25% of the Hif isolates were positive for the gene encoding the ZitB transporter. Only two isolates carried the molC gene, but were negative for molA, indicating a partial deletion of the mol operon. In addition, all Hif isolates exhibited a full or partial deletion of the histidine biosynthesis operon. Our data thus suggested that the pattern of unique or missing genes in Hif KR494 was consistent in different Hif isolates. There was no specific genetic features that distinguished isolates from different geographical areas, or that could predict clinical severity. The latter observation also highlighted the importance of host factors in clinical disease.
Table 6.
Distribution of genes absent in Hif KR494 and other Hif clinical isolates
| Locus a | Description/gene b | Serotype f clinical isolates | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KR494 | G19 | G20 | K238 | L11 | L16 | L21 | L22 | L24 | L25 | L29 | L45 | L50 | L59 | M1 | M10 | M14 | M29 | M54 | S208 | S229 | No. of isolate c | Hib MinnA | NTHi 3655 | ||
| Histidine biosynthesis | |||||||||||||||||||||||||
| HI0468 | hisG | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | + | - | 2 | + | + |
| HI0469 | hisD | - | + | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | 3 | + | + |
| HI0470 | hisC | - | + | + | - | + | - | + | - | + | - | + | - | + | + | + | + | + | - | - | + | - | 12 | + | + |
| HI0472 | hisH | - | - | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | 2 | + | + |
| Anaerobic fermentation of L-ascorbate | |||||||||||||||||||||||||
| HI1024 | ulaD | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | 1 | -d | -d |
| Folate biosynthesis | |||||||||||||||||||||||||
| HI1190 | PTS | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | 1 | + | + |
| Zinc and secondary molybdenum transporter | |||||||||||||||||||||||||
| HIB_07090 | zitB | - | - | + | - | - | - | - | - | - | - | - | - | - | + | + | - | - | - | - | + | + | 5 | + | + |
| HI1470 | molC | - | - | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | 2 | + | -d |
| HI1472 | molA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | + | -d |
| hmg locus involved in LOS glycoform | |||||||||||||||||||||||||
| HI0871 | siaA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | + | + |
| HI0872 | wbaP | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | + | + | |
| No. of CDSs present | 0 | 2 | 5 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 3 | 5 | 3 | 1 | 0 | 0 | 3 | 1 | 10 | 8 | ||
aLocus tags were based upon the genome of Rd Kw20 except HIB_07090 is based on Hib 10810. For PCR targeting the contiguous loci, only the first locus is shown. Full information of the target genes, amplicon size and PCR conditions is described in Additional file 1.
bAbbreviations: PTS, 6-pyruvoyl tetrahydrobiopterin synthase in folate biosynthesis.
cTotal number of Hif clinical isolates (excluding KR494) with genes that were absent in Hif KR494.
dCDSs that were present in Rd Kw20 but not in NTHi 3655 or Hib MinnA.
+PCR positive (CDS present); -PCR negative (CDS absent).
Histidine biosynthesis defect and kanamycin resistance of Hif KR494
To confirm the relevance of the genetic findings, a series of functional experiments were performed. Since histidine is crucial for bacterial growth, we wanted to know whether the Hif gene deletions of the his operon would interfere with histidine biosynthesis and cell growth. We performed a histidine auxotrophic assay and found that all Hif isolates including KR494 were defective in growth when cultured in histidine-depleted medium (w/o-His) (Additional file 5). In contrast, the isolates grew well in histidine-supplemented (w-His) medium, whereas the positive controls Hib MinnA and NTHi 3655 readily grew in both w-His and w/o-His media. Our experiments thus showed that the inability to catalyze the de novo biosynthesis of histidine made Hif KR494 and other Hif isolates dependent on an external histidine source, excluding the possibility of any alternative histidine synthesis pathway.
Since H. influenzae kanamycin resistance is rarely reported, we also tested the Hif isolates for kanamycin susceptibility. We found that isolates containing the KNTase homologue (HifGL_000799-HifGL_000800) had kanamycin MIC of 4 μg/ml that was 4-fold higher than the Hib MinnA (1 μg/ml) and 8-fold higher than NTHi 3655 (0.5 μg/ml), which both lacked the kanamycin resistance genes (Additional file 5).
Multiple genome alignments of closely-related human Haemophilusspp
Haemophilus influenzae, H. aegyptius and H. haemolyticus belong to the cluster of ‘Haemophilus sensu stricto’ (Hss) [62, 63], whereas H. parainfluenzae is located in a distinct cluster called Parainfluenzae, but is the closest neighbor species to the Hss group. Importantly, the Hss and Parainfluenzae groups share the same host, and thus are functionally closely related. Taking into account that a number of Hif KR494 unique genes are exclusively homologous to H. aegyptius, H. haemolyticus and H. parainfluenzae, a cross species genomic comparison was performed with these selected strains (Table 2). ACT alignment revealed an extensive divergence in gene order (Figure 5), indicating several gene rearrangements between the genomes of Hif KR494 and the related species. The Hif KR494 genome had a moderate level of synteny with the H. aegyptius ATCC 11116, but less with H. haemolyticus M21639 or H. parainfluenzae ATCC 33392. At a cut-off of 85% protein sequence similarity, a high number of genes from Hif KR494, that is, 1487 CDSs (85.4% of the total CDSs) were shared with H. aegyptius. This was reduced to 1451 CDSs (83.3%) with H. haemolyticus and 1366 CDSs (78.4%) with H. parainfluenzae (Table 2). Our pangenomic data analyses thus implied a higher genome similarity between Hif and H. aegyptius than with H. haemolyticus and H. parainfluenzae, respectively. This picture confirmed previous phylogenetic studies on human Haemophilus spp. [62, 63]. In total, 1295 CDSs of Hif KR494 (74.3%) were in common with the related species, whereas 190 CDSs were less conserved mainly within the RgDFs (Additional file 6 and Additional file 7). Finally, a global pangenomic analysis revealed that 133 of the Hif KR494 unique genes were less conserved or absent in the H. influenzae reference strains, H. aegyptius, H. haemolyticus and H. parainfluenzae (Table 2), which represents the universal unique genome of Hif KR494 (Additional file 8).
Figure 5.
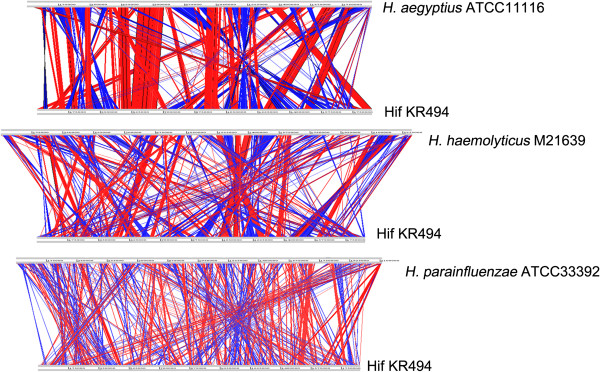
A cross species genomic comparison of H. influenzae type f KR494 and human Haemophilus spp. ACT view of synteny between genomes of Hif KR494 and H. aegyptius ATCC11116 (upper panel), H. haemolyticus M21639 (middle) and H. parainfluenzae ATCC33392 (lower panel). Respective genome designations are indicated on the right hand side of each genome line. Direct and inverted synteny between individual ORF (not indicated here) of the comparing genomes are shown in red and blue, respectively.
Discussion
To define the genomic factors that may contribute to the virulence traits of the emerging pathogen H. influenzae type f, we initially conducted a pangenomic analysis with three other complete genomes representing encapsulated and nontypeable H. influenzae. Since Hif isolates are suggested to be clonal [11, 18, 64], we used the recently sequenced Hif KR494, a necrotizing myositis isolate, as a primary model to identify genes potentially associated with virulence. We show that the divergence in the Hif KR494 genome is likely due to small-scale genetic rearrangements involving both gene acquisition (insertion) and gene loss in addition to some minor inversions. Importantly, the majority of genes identified as essential for the pathogenicity of H. influenzae were conserved and intact in Hif KR494 [65]. These include genes implicated in nutrient acquisition, LOS biosynthesis/modification and oxidative stress responses. Genes encoding for proteins mediating interactions with airway epithelial cells were also conserved [66, 67], reassuring Hif-dependent adhesion and subsequent colonization of the human host. Metabolic and growth requirements for H. influenzae have been very well studied, but solely based upon the strain Rd Kw20. Those analyses revealed at least 461 metabolic reactions operating on 367 internal metabolites and 84 external metabolites [68, 69]. Given that the Hif KR494 genome contains most but not all of the metabolic enzymes of Rd Kw20, we postulate similar metabolic machinery in Hif. A serotype-specific metabolism remains, however, to be determined.
Gene acquisition in H. influenzae, in particular the nontypeable strains, has been associated with bacterial genetic adaptation and is mainly attributed to horizontal DNA transfer [26, 34–36]. Like other mucosal pathogens, H. influenzae is naturally competent, and the ability to take up DNA is facilitated by the presence of DNA sequence uptake signals (USSs) [70]. There are 1496 USSs identified in the Hif KR494 genome (1566 in Hib 10810, 1471 in Rd Kw20, 1482 in NTHi 3655). The established mechanisms of horizontal DNA transfer in H. influenzae include transduction and infection by Haemophilus bacteriophages, DNA transformation, transconjugation of the ICEHin1056 family and, finally, integration of genetic islands [54–56, 71–74]. We identified a Hif accessory genome that in several aspects, i.e., surface structure, energy conversions and metabolic pathways, may contribute to the unique features of type f strains (Figure 3B). While the majority of known H. influenzae-associated virulence genes were conserved, the Hif genome contained additional putative virulence genes that were not identified in the H. influenzae reference strains. Several of these unique Hif KR494 CDSs had limited homology to the published H. influenzae accessory gene pool and exhibited atypical GC content (31.5%-48.5%) (Table 3). This clearly suggested acquisition through horizontal gene transfer. Notably, unique genes with slightly altered GC content may have been acquired from species with GC content similar to that of H. influenzae. The unique genes may have been introduced to the Hif genome through direct DNA uptake, transposed as a composite DNA, via prophage infection or integration as genetic islands. The presence of adjacent prophage islands and the abundance of mobile genetic elements and USS sites accompanying the unique genes suggest such events, mainly at RgDFs (Table 3). This implies that the Hif KR494 genome had a relatively uncomplicated gene acquisition mechanism that is not dependent on plasmids nor ICE elements since the latter two DNA components were not found in the Hif KR494.
Gene duplications have been shown to affect pathogenicity in some H. influenzae strains. Hib variants containing additional capsule loci are generally more virulent and the pathogenicity has been suggested to be proportionate to the gene copy number and amount of capsule deposited at the surface [75, 76]. Therefore, Hif KR494 genes that are associated with gene duplication were carefully determined. We postulate that a multiplication of metabolic genes may improve the Hif metabolism and energy production to enhance fitness during infection as described for several pathogenic fungi and the genetically related H. influenzae biogroup aegyptius and H. aegyptius[65, 77–79]. Additional paralogous genes associated with virulence such as the Sap transporter, fimbriae, heme transfer proteins and kanamycin resistance proteins may also increase virulence, and needs to be further studied.
We found a “duplication” of the Sap transporter, i.e., SapABCDFZ and Sap2ABCDF, that may enhance the bacterial resistance against AMPs, increase the heme acquisition, and promote homeostasis in potassium uptake and interactions with epithelial cells [38, 39, 80]. The specific type of AMPs species targeted by the periplasmic solute binding protein Sap2A (a H. parainfluenzae SapA homologue) in Hif, is unknown and may be different from the H. influenzae SapA due to sequence heterogeneity.
The co-existence of different fimbriae types has been reported in H. aegyptius and H. influenzae biogroup aegyptius (conjunctivitis and BPF clones) [29, 41, 44]. NTHi and non-f capsulated H. influenzae, however, have only a single type of fimbriae locus (hif). Thus, this is the first report suggesting the presence of two distinct fimbriae loci (hif and aef) in Hif, resulting in a genotype similar to H. aegyptius. Although the role of Aef in virulence is not fully clear, the functionality of HifACDE fimbriae in Hif strains, which generally lack the chaperon subunit HifB may be compensated by the Aef3abcdef [29, 44]. The existence of Aef3 in the Hif genome offers an explanation to the haemagglutination phenotype of Hif that was observed despite the absence of the subunit hifB [12]. Interestingly, it is suggested that abundant pili/fimbriae (Hif and Aef) facilitate/promote initial colonization of the human nasopharynx, but are down-regulated prior to subsequent systemic invasion to prevent immune recognition [43].
Topology analysis with PSORTb suggested that the heme-binding protein HifGL_001444 is a periplasmic protein (data not shown). It may function as an alternative periplasmic transporter in addition to the previously described HbpA and HipA/DppA facilitating the transport of heme/iron across the periplasm to the cytoplasmic membrane transporter [49]. Moreover, the region spanning amino acid residues 52 and 322 comprises domain SBP_bac_8 (Pfam 13416), suggested that this protein belongs to the AfuA family (COG1840), a periplasmic component of ABC-type Fe3+ transport system. However, at the protein level HifGL_001444 shares only 33% of sequence homology with the AfuA of Rd Kw20. In addition, the HutZ (HifGL_0016364) homologue in Vibro cholerae was proposed as a heme storage protein and important for heme trafficking across the membrane to heme-containing proteins [81]. Thus, both HifGL_001444 and HutZ may confer a unique heme utilization machinery on Hif compared to other serotypes that lack these genes.
Intriguingly, all 20 Hif isolates screened in the present study contain the KNTase-related genes (HifGL_000799-HifGL_000800), and consequently are more resistant to kanamycin compared to Hib MinnA and NTHi 3655 that do not have these genes. This information is valuable for clinicians, since aminoglycosides are often used in the treatment of severe sepsis. More experiments are, however, needed to show the significance of KNTase-related genes in antimicrobial resistance.
Gene loss/deletion may also be beneficial for Hif although opposing the evolutionary force of gene acquisition. In fact, this phenomenon has been reported in H. influenzae biogroup aegyptius and other human pathogens such as Francisella tularensis, Yersinia pestis and Shigella spp., Rickettsia spp. and Mycobacterium spp. [77, 82–84]. Gene loss in microbial pathogens is principally caused by i) adaptation to a more specific niche of which certain gene products become unnecessary and, ii) inactivation/elimination of antivirulence genes (AVG) that is incompatible to newly acquired virulence factors [84]. However, the AVG concept has not yet been reported for H. influenzae. The majority of the deleted genes in Hif KR494 are not essential for establishing infection in vivo[65]. The Hif KR494 genome lacked three putative virulence genes involved in mouse pulmonary infection (pdgX, stress defense) and infant rat bactermia (rfbP and rfbB for LOS biosynthesis) [65].
The histidine biosynthesis pathway is of particular interest since it cannot be found in Hif KR494 or other Hif isolates examined in the present study (Tables 4 and 6). Since Hif is not a pathogen associated with acute otitis media this observation fits with the hypothesis that the histidine pathway is a survival strategy for NTHi isolates to cope with the limited histidine conditions in the middle ear [24]. The auxotrophic Hif phenotype, however, may not interfere with bacterial virulence as described in other his deficient species such as Helicobacter pylori 26695 and Mycobacterium genitalium G-37 [85, 86]. It is plausible that the histidine-rich environment in the throat may support initial colonization of Hif as reported in other his-negative auxotrophic throat commensals prior to its migration into systemic organs [24]. Since Hif KR494 was isolated from both blood and muscle tissue [9], its survival during subsequent invasive infection might depend on the uptake of exogenous histidine from surrounding niches. This is supported by the availability of free histidine or in the form of histamine and histidine-rich glycoproteins in blood and tissues [87–89]. We suggest that the absent histidine biosynthesis pathway may be one of the factors rendering Hif a less effective colonizer of the human airway, and may offer an explanation of why Hif is found in invasive disease rather than in respiratory tract infections. This speculation is consistent with the finding of gene loss events (i.e., loss of genes involved in energy metabolism and nutrient transport) in BPF-related H. influenzae biogroup aegyptius HK1212. This particluar strain had a putative genome evolution as driving force towards a higher dependency on the host energy and metabolites for a secure adaptation to the host environment [77].
The quantities of molybdate are reported in the ~100-1000 nM range in whole blood [90]. For Hif, the high affinity ModABCE system (Kd = 10 nM-1 μM) might be preferable, whereas the low affinity MolABCD system (Kd = ~100 μM) becomes superfluous [60, 61]. Genes involved in the LOS biosynthesis were altered since the entire hmg responsible for different LOS glycoforms was deleted. Moreover, siaA encoded within the hmg locus is required for biofilm formation of NTHi otitis media isolates [59, 91]. Mucosal pathogens are generally protected by a biofilm that promotes local colonization, and consequently prevents detachment and transmission from the infection site [92]. While we cannot rule out the impact of hmg gene deletion for Hif virulence, we hypothesized whether the gene loss may cause defects in biofilm formation, aiding to bacterial systemic dissemination from mucosal sites, as seen in the hypervirulent Neisseria meningitidis[93].
In addition to Hif strains that are monophyletic regarding the 7 housekeeping genes used in MLST [18], both Rodriguez et al. and Watson et al.[11, 12] found a homogenous distribution of the known virulence genes (hsf, hif, hap and lic2BC). Based on these studies, it was suspected that Hif isolates are generally clonal. The analysis of the unique genomic features identified in the Hif KR494 genome in 20 clinical Hif strains confirmed this assumption (Tables 5 and 6). In addition, MLST showed that all Hif isolates tested from different parts of Sweden were of sequence type (ST) 124 [3]. Most clinical Hif strains displayed a near-perfect match to Hif KR494, varying only in one to four genes. Our result is also congruent with previous studies supporting the limited genetic diversity of serotype f despite being implicated in a wide variety of clinical severities and infection sites.
The Hif KR494 genome exerted a H. aegyptius-like genotypic characteristic, i.e., 85.4% of the total Hif KR494 CDSs were homologous to H. aegyptius ATCC1116. This is proximate to the degree of similarity (85.8%) observed between Hif KR494 and Hib 10810, and was more conserved than Hid Rd Kw20 (83.7%). Although two prior phylogenetic investigations revealed that H. aegyptius ATCC1116 is genetically the closest Haemophilus species to H. influenzae based upon the nontypeable HK389 and typeable P1557 (serotype a) and P1227 (serotype b), Hif was not included in those studies and the precise relationship thus needs to be further elucidated [62, 63]. Nonetheless within the species of H. influenzae, phylogenetical analysis (based on MLST) by Meats et al.[18] together with a recent phylogenomic study (pairwise alignment of partial-genome sequences from 70 nontypeable and capsulated (a-f; except d) strains) revealed that serotype f is genetically closely related to serotype a and e [18, 74]. This is interesting since both serotype a and e were recently reported to be potentially invasive [4, 94, 95]. However, attempts to include type a and e in our current study was hampered by the absence of the reported genome sequences in public databases [74]. The genetical diversity of the Hif KR494 genome (Table 3), however, is limited to orthologs within the Hin subclade (H. influenzae, H. aegyptius, H. parainfluenzae, H. haemolyticus, A. actinomycetemcomitans, P. multocida, M. succiniciproducens, and H. somnus) of the Pasteurellaceae family. This may be explained by the findings of Redfield et al. [96], which revealed a DNA uptake specificity of the Hin subclade that is preferentially dependent on H. influenzae USS consensus sequence.
Conclusions
The comparative analyses have identified unique features of the Hif KR494 genome that may increase the understanding of Hif pathogenesis. Gene rearrangements involving inversion, insertion and deletion were evident despite a large similarity in genomic organization between Hif and other previously sequenced serotypes. Our analysis resulted in a wide compilation of gene functions unique to Hif. The gene products involved in metabolism and virulence that are not found in other serotypes may also contribute to the Hif pathogenicity associated with invasive disease. The in silico analysis, however, did not make it possible to determine the specific virulence factors that may explain differences between the analyzed Haemophilus species. It remains to elucidate whether these newly discovered Hif genes can be used as biomarkers for serotype differentiation or targets for antimicrobial drug design.
Authors’ information
YCS, FR and KR are research scientists at Medical Microbiology, Department of Laboratory Medicine Malmö, Lund University, Jan Waldenströms gata 59, SE-205 02 Malmö, Sweden. FH is a bioinformatics student at Institute of Computer Science, Department of Mathematics and Computer Science, Friedrich-Schiller-University of Jena, PF 07737, Jena, Germany.
Electronic supplementary material
Additional file 1: List of primers and PCR conditions used in present study. (PDF 13 KB)
Additional file 2: ACT view of multiple genome alignment among human-related Haemophilus spp. Respective genome designations are indicated on the right hand side of each genome line. Forward (+) and complement (−) strands of individual genomes are indicated in the grey genome lines. Genomes are shown in full length and drawn to scale. Direct and inverted synteny between individual ORF (not indicated here) of the compared genomes are shown in red and blue, respectively. The level of amino acid similarity is represented by color shading with ascending saturation and indicates higher similarity. (PDF 243 KB)
Additional file 3: List of loci encoded at RgD F s of the Hif KR494 genome. (PDF 7 KB)
Additional file 4: Predicted genetic islands of serotype f (Gif KR494 ) defined in the Hif KR494 genome. (PDF 10 KB)
Additional file 5: Histidine auxotrophic and antibiotics susceptibility assay. (PDF 54 KB)
Additional file 6: Cross species genomic comparative of the closely-related human Haemophilus spp. A cross species genomic comparison of H. influenzae type f KR494 and human Haemophilus spp (H. aegyptius ATCC 11116, H. haemolyticus M21639 and H. parainfluenzae ATCC 33392). COG distribution and functionality classification of Hif KR494 CDSs that is (A) commonly shared and (B) unique CDSs in regards to the related Haemophilus spp. (PDF 107 KB)
Additional file 7: Map of RgD F in the H. influenzae type f KR494 genome in relative to the closely-related human Haemophilus species. Circular representation of protein conservation between Hif KR494 and the reference species was visualized using DNA plotter. From the outside in, the outer circle shows the genome length of Hif KR494 with position markers. The second circle shows the total ORFs of KR494 genome predicted on both forward and reverse strands. Common and unique ORFs in relative to the reference species are colored in blue and magenta, respectively. Phage-related ORFs are marked in yellow and orange. The third to fifth circles represent the distribution of individual ORF with high homology (≥85% similarity) (in red) to the corresponding ORF in reference species H. aegyptius ATCC11116, H. haemolyticus M21639 and H. parainfluenzae ATCC33392, respectively. Gaps between the conserved ORFs represent RgDF between Hif KR494 and the compared species, and were denoted as RgDF1 to RgDF7 (marked with green lines). GC plot and GC skew of the Hif KR494 genome are shown in the sixth and seventh circle, respectively. The genome of Hif KR494 was less conserved with H. aegyptius, H. haemolyticus and H. parainfluenzae at the RgDF1, 2, 3, 5, 6 and 7. The RgDF7 comprises xylFGH and xylAB operons (HifGL_000770-HifGL_000777) that are involved in xylose uptake and metabolism through the pentose phosphate pathway. This indicated that H. aegyptius, H. haemolyticus and H. parainfluenzae lacked a xylose metabolism system that is, however, conserved in H. influenzae. (PDF 323 KB)
Additional file 8: Total genomic comparison of Hif KR494 with H. influenzae reference strains, H. aegyptius, H. haemolyticus and H. parainfluenzae. Unique genes (133 CDSs) of Hif KR494 that consistently lacked homology with any of the aligned species were delineated based on the COG database. Notably, when genes of unknown function were excluded, most of the universal unique CDSs of Hif KR494 were phage-related products, followed by extracellular structures. The data represent the universal gene feature of Hif KR494. (PDF 77 KB)
Acknowledgements
This work was supported by grants from the Alfred Österlund, the Anna and Edwin Berger, Greta and Johan Kock, the Gyllenstiernska Krapperup, Åke Wiberg, Hans Hierta, the Swedish Medical Research Council (grant number 521-2010-4221, http://www.vr.se), the Cancer Foundation at the University Hospital in Malmö, the Physiographical Society (Forssman’s Foundation), and Skåne County Council’s research and development foundation. We thank Mr. Farshid Jalalvand for the constructive comments during the preparation of this manuscript.
Abbreviations
- ACT
Artemis comparative tools
- AVG
Antivirulence gene
- CDS
Coding sequence
- COPD
Chronic obstructive pulmonary diseases
- COG
Cluster of orthologous groups
- Hib
H. influenzae type b
- Hid
H. influenzae type d
- Hif
H. influenzae type f
- LOS
Lipooligosaccharide
- MIC
Minimal inhibitory concentration
- NTHi
Nontypeable H. influenzae
- ORF
Open reading frame
- RgDF
Hif-region of difference
- TAA
Trimeric autotransporter.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YCS, FR and KR designed the study and prepared the manuscript. SYC and FH performed computational comparative genomics. FH wrote Perl script. FR performed PCR screening and evaluation of genotypic conservation among Hif isolates. SYC did histidine auxotrophic and kanamycin resistance assay. All authors read and agreed with the final manuscript.
Contributor Information
Yu-Ching Su, Email: Shanice_YC.Su@med.lu.se.
Fredrik Resman, Email: Fredrik.Resman@med.lu.se.
Franziska Hörhold, Email: franziska.hoerhold@uni-jena.de.
Kristian Riesbeck, Email: kristian.riesbeck@med.lu.se.
References
- 1.Agrawal A, Murphy TF. Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. J Clin Microbiol. 2011;49:3728–3732. doi: 10.1128/JCM.05476-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thanavala Y, Lugade AA. Role of nontypeable Haemophilus influenzae in otitis media and chronic obstructive pulmonary disease. Adv Otorhinolaryngol. 2011;72:170–175. doi: 10.1159/000324785. [DOI] [PubMed] [Google Scholar]
- 3.Resman F, Ristovski M, Ahl J, Forsgren A, Gilsdorf JR, Jasir A, Kaijser B, Kronvall G, Riesbeck K. Invasive disease caused by Haemophilus influenzae in Sweden 1997–2009: evidence of increasing incidence and clinical burden of non-type b strains. Clin Microbiol Infect. 2011;17:1638–1645. doi: 10.1111/j.1469-0691.2010.03417.x. [DOI] [PubMed] [Google Scholar]
- 4.Ladhani SN, Collins S, Vickers A, Litt DJ, Crawford C, Ramsay ME, Slack MP. Invasive Haemophilus influenzae serotype e and f disease, England and Wales. Emerg Infect Dis. 2012;18:725–732. doi: 10.3201/eid1805.111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacNeil JR, Cohn AC, Farley M, Mair R, Baumbach J, Bennett N, Gershman K, Harrison LH, Lynfield R, Petit S, et al. Current epidemiology and trends in invasive Haemophilus influenzae disease–United States, 1989–2008. Clin Infect Dis. 2011;53:1230–1236. doi: 10.1093/cid/cir735. [DOI] [PubMed] [Google Scholar]
- 6.Rubach MP, Bender JM, Mottice S, Hanson K, Weng HY, Korgenski K, Daly JA, Pavia AT. Increasing incidence of invasive Haemophilus influenzae disease in adults, Utah, USA. Emerg Infect Dis. 2011;17:1645–1650. doi: 10.3201/eid1709.101991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronit A, Berg RM, Bruunsgaard H, Plovsing RR. BMJ Case Rep. 2013. Haemophilus influenzae type f meningitis in a previously healthy boy; p. bcr2013008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urwin G, Krohn JA, Deaver-Robinson K, Wenger JD, Farley MM. Invasive disease due to Haemophilus influenzae serotype f: clinical and epidemiologic characteristics in the H. influenzae serotype b vaccine era. Clin Infect Dis. 1996;22:1069–1076. doi: 10.1093/clinids/22.6.1069. [DOI] [PubMed] [Google Scholar]
- 9.Resman F, Svensjo T, Unal C, Cronqvist J, Brorson H, Odenholt I, Riesbeck K. Necrotizing myositis and septic shock caused by Haemophilus influenzae type f in a previously healthy man diagnosed with an IgG3 and a mannose-binding lectin deficiency. Scand J Infect Dis. 2011;43:972–976. doi: 10.3109/00365548.2011.589079. [DOI] [PubMed] [Google Scholar]
- 10.Suarez CJ, Glover WA, Cowan J, Smith A, Clarridge JE., 3rd Mycotic aneurysm of the abdominal aorta caused by Haemophilus influenzae type f. J Med Microbiol. 2013;62:658–660. doi: 10.1099/jmm.0.055228-0. [DOI] [PubMed] [Google Scholar]
- 11.Watson ME, Jr, Nelson KL, Nguyen V, Burnham CA, Clarridge JE, Qin X, Smith AL. Adhesin genes and serum resistance in Haemophilus influenzae type f isolates. J Med Microbiol. 2013;62:514–524. doi: 10.1099/jmm.0.052175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez CA, Avadhanula V, Buscher A, Smith AL, St Geme JW, 3rd, Adderson EE. Prevalence and distribution of adhesins in invasive non-type b encapsulated Haemophilus influenzae. Infect Immun. 2003;71:1635–1642. doi: 10.1128/IAI.71.4.1635-1642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satola SW, Schirmer PL, Farley MM. Genetic analysis of the capsule locus of Haemophilus influenzae serotype f . Infect Immun. 2003;71:7202–7207. doi: 10.1128/IAI.71.12.7202-7207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campos J, Roman F, Perez-Vazquez M, Aracil B, Oteo J, Cercenado E, Spanish Study Group for Hitf Antibiotic resistance and clinical significance of Haemophilus influenzae type f. J Antimicrob Chemother. 2003;52:961–966. doi: 10.1093/jac/dkh004. [DOI] [PubMed] [Google Scholar]
- 15.Su YC, Hornold F, Singh B, Riesbeck K. Complete genome sequence of encapsulated Haemophilus influenzae type f KR494, an invasive isolate that caused necrotizing myositis. Genome Announce. 2013;1:e00470–13. doi: 10.1128/genomeA.00470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munson R, Jr, Grass S. Purification, cloning, and sequence of outer membrane protein P1 of Haemophilus influenzae type b. Infect Immun. 1988;56:2235–2242. doi: 10.1128/iai.56.9.2235-2242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musser JM, Barenkamp SJ, Granoff DM, Selander RK. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun. 1986;52:183–191. doi: 10.1128/iai.52.1.183-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meats E, Feil EJ, Stringer S, Cody AJ, Goldstein R, Kroll JS, Popovic T, Spratt BG. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J Clin Microbiol. 2003;41:1623–1636. doi: 10.1128/JCM.41.4.1623-1636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carver TJ, Rutherford KM, Berriman M, Rajandream MA, Barrell BG, Parkhill J. ACT: the Artemis Comparison Tool. Bioinformatics. 2005;21:3422–3423. doi: 10.1093/bioinformatics/bti553. [DOI] [PubMed] [Google Scholar]
- 21.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273–279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream MA, Barrell B. Artemis: sequence visualization and annotation. Bioinformatics. 2000;16:944–945. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 23.Carver T, Thomson N, Bleasby A, Berriman M, Parkhill J. DNAPlotter: circular and linear interactive genome visualization. Bioinformatics. 2009;25:119–120. doi: 10.1093/bioinformatics/btn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juliao PC, Marrs CF, Xie J, Gilsdorf JR. Histidine auxotrophy in commensal and disease-causing nontypeable Haemophilus influenzae. J Bacteriol. 2007;189:4994–5001. doi: 10.1128/JB.00146-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herriott RM, Meyer EY, Vogt M, Modan M. Defined medium for growth of Haemophilus influenzae. J Bacteriol. 1970;101:513–516. doi: 10.1128/jb.101.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogg JS, Hu FZ, Janto B, Boissy R, Hayes J, Keefe R, Post JC, Ehrlich GD. Characterization and modeling of the Haemophilus influenzae core and supragenomes based on the complete genomic sequences of Rd and 12 clinical nontypeable strains. Genome Biol. 2007;8:R103. doi: 10.1186/gb-2007-8-6-r103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleischmann RD, Adams MD, White O, Clayton RA, Kirkness EF, Kerlavage AR, Bult CJ, Tomb JF, Dougherty BA, Merrick JM, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 28.Whitby PW, Seale TW, Morton DJ, VanWagoner TM, Stull TL. Characterization of the Haemophilus influenzae tehB gene and its role in virulence. Microbiology. 2010;156:1188–1200. doi: 10.1099/mic.0.036400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strouts FR, Power P, Croucher NJ, Corton N, van Tonder A, Quail MA, Langford PR, Hudson MJ, Parkhill J, Kroll JS, Bentley SD. Lineage-specific virulence determinants of Haemophilus influenzae biogroup aegyptius. Emerg Infect Dis. 2012;18:449–457. doi: 10.3201/eid1803.110728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norskov-Lauritsen N, Overballe MD, Kilian M. Delineation of the species Haemophilus influenzae by phenotype, multilocus sequence phylogeny, and detection of marker genes. J Bacteriol. 2009;191:822–831. doi: 10.1128/JB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison A, Dyer DW, Gillaspy A, Ray WC, Mungur R, Carson MB, Zhong H, Gipson J, Gipson M, Johnson LS, et al. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J Bacteriol. 2005;187:4627–4636. doi: 10.1128/JB.187.13.4627-4636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Challacombe JF, Duncan AJ, Brettin TS, Bruce D, Chertkov O, Detter JC, Han CS, Misra M, Richardson P, Tapia R, et al. Complete genome sequence of Haemophilus somnus (Histophilus somni) strain 129Pt and comparison to Haemophilus ducreyi 35000HP and Haemophilus influenzae Rd. J Bacteriol. 2007;189:1890–1898. doi: 10.1128/JB.01422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tatusov RL, Koonin EV, Lipman DJ. A genomic perspective on protein families. Science. 1997;278:631–637. doi: 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- 34.Fernaays MM, Lesse AJ, Sethi S, Cai X, Murphy TF. Differential genome contents of nontypeable Haemophilus influenzae strains from adults with chronic obstructive pulmonary disease. Infect Immun. 2006;74:3366–3374. doi: 10.1128/IAI.01904-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen K, Antalis P, Gladitz J, Sayeed S, Ahmed A, Yu S, Hayes J, Johnson S, Dice B, Dopico R, et al. Identification, distribution, and expression of novel genes in 10 clinical isolates of nontypeable Haemophilus influenzae. Infect Immun. 2005;73:3479–3491. doi: 10.1128/IAI.73.6.3479-3491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie J, Juliao PC, Gilsdorf JR, Ghosh D, Patel M, Marrs CF. Identification of new genetic regions more prevalent in nontypeable Haemophilus influenzae otitis media strains than in throat strains. J Clin Microbiol. 2006;44:4316–4325. doi: 10.1128/JCM.01331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eutsey RA, Hiller NL, Earl JP, Janto BA, Dahlgren ME, Ahmed A, Powell E, Schultz MP, Gilsdorf JR, Zhang L, et al. Design and validation of a supragenome array for determination of the genomic content of Haemophilus influenzae isolates. BMC Genomics. 2013;14:484. doi: 10.1186/1471-2164-14-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mason KM, Bruggeman ME, Munson RS, Bakaletz LO. The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol Microbiol. 2006;62:1357–1372. doi: 10.1111/j.1365-2958.2006.05460.x. [DOI] [PubMed] [Google Scholar]
- 39.Vogel AR, Szelestey BR, Raffel FK, Sharpe SW, Gearinger RL, Justice SS, Mason KM. SapF-mediated heme-iron utilization enhances persistence and coordinates biofilm architecture of Haemophilus. Front Cell Infect Microbiol. 2012;2:42. doi: 10.3389/fcimb.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis GS, Sandstedt SA, Patel M, Marrs CF, Gilsdorf JR. Use of bexB to detect the capsule locus in Haemophilus influenzae. J Clin Microbiol. 2011;49:2594–2601. doi: 10.1128/JCM.02509-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilsdorf JR, McCrea KW, Marrs CF. Role of pili in Haemophilus influenzae adherence and colonization. Infect Immun. 1997;65:2997–3002. doi: 10.1128/iai.65.8.2997-3002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Read RC, Wilson R, Rutman A, Lund V, Todd HC, Brain AP, Jeffery PK, Cole PJ. Interaction of nontypable Haemophilus influenzae with human respiratory mucosa in vitro. J Infect Dis. 1991;163:549–558. doi: 10.1093/infdis/163.3.549. [DOI] [PubMed] [Google Scholar]
- 43.Mhlanga-Mutangadura T, Morlin G, Smith AL, Eisenstark A, Golomb M. Evolution of the major pilus gene cluster of Haemophilus influenzae. J Bacteriol. 1998;180:4693–4703. doi: 10.1128/jb.180.17.4693-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Read TD, Dowdell M, Satola SW, Farley MM. Duplication of pilus gene complexes of Haemophilus influenzae biogroup aegyptius. J Bacteriol. 1996;178:6564–6570. doi: 10.1128/jb.178.22.6564-6570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biegel E, Muller V. Bacterial Na + −translocating ferredoxin:NAD + oxidoreductase. Proc Natl Acad Sci USA. 2010;107:18138–18142. doi: 10.1073/pnas.1010318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandrasegaran S, Lunnen KD, Smith HO, Wilson GG. Cloning and sequencing the HinfI restriction and modification genes. Gene. 1988;70:387–392. doi: 10.1016/0378-1119(88)90210-7. [DOI] [PubMed] [Google Scholar]
- 47.Gerratana B, Cleland WW, Reinhardt LA. Regiospecificity assignment for the reaction of kanamycin nucleotidyltransferase from Staphylococcus aureus. Biochemistry. 2001;40:2964–2971. doi: 10.1021/bi0025565. [DOI] [PubMed] [Google Scholar]
- 48.Porter VR, Green KD, Zolova OE, Houghton JL, Garneau-Tsodikova S. Dissecting the cosubstrate structure requirements of the Staphylococcus aureus aminoglycoside resistance enzyme ANT(4′) Biochem Biophys Res Commun. 2010;403:85–90. doi: 10.1016/j.bbrc.2010.10.119. [DOI] [PubMed] [Google Scholar]
- 49.Whitby PW, Seale TW, VanWagoner TM, Morton DJ, Stull TL. The iron/heme regulated genes of Haemophilus influenzae: comparative transcriptional profiling as a tool to define the species core modulon. BMC Genomics. 2009;10:6. doi: 10.1186/1471-2164-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morton DJ, Seale TW, Vanwagoner TM, Whitby PW, Stull TL. The dppBCDF gene cluster of Haemophilus influenzae: Role in heme utilization. BMC Res Notes. 2009;2:166. doi: 10.1186/1756-0500-2-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morton DJ, Madore LL, Smith A, Vanwagoner TM, Seale TW, Whitby PW, Stull TL. The heme-binding lipoprotein (HbpA) of Haemophilus influenzae: role in heme utilization. FEMS Microbiol Lett. 2005;253:193–199. doi: 10.1016/j.femsle.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 52.May BJ, Zhang Q, Li LL, Paustian ML, Whittam TS, Kapur V. Complete genomic sequence of Pasteurella multocida, Pm70. Proc Natl Acad Sci USA. 2001;98:3460–3465. doi: 10.1073/pnas.051634598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juhas M, Power PM, Harding RM, Ferguson DJ, Dimopoulou ID, Elamin AR, Mohd-Zain Z, Hood DW, Adegbola R, Erwin A, et al. Sequence and functional analyses of Haemophilus spp. genomic islands. Genome Biol. 2007;8:R237. doi: 10.1186/gb-2007-8-11-r237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohd-Zain Z, Turner SL, Cerdeno-Tarraga AM, Lilley AK, Inzana TJ, Duncan AJ, Harding RM, Hood DW, Peto TE, Crook DW. Transferable antibiotic resistance elements in Haemophilus influenzae share a common evolutionary origin with a diverse family of syntenic genomic islands. J Bacteriol. 2004;186:8114–8122. doi: 10.1128/JB.186.23.8114-8122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergman NH, Akerley BJ. Position-based scanning for comparative genomics and identification of genetic islands in Haemophilus influenzae type b. Infect Immun. 2003;71:1098–1108. doi: 10.1128/IAI.71.3.1098-1108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang CC, Gilsdorf JR, DiRita VJ, Marrs CF. Identification and genetic characterization of Haemophilus influenzae genetic island 1. Infect Immun. 2000;68:2630–2637. doi: 10.1128/IAI.68.5.2630-2637.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fani R, Mori E, Tamburini E, Lazcano A. Evolution of the structure and chromosomal distribution of histidine biosynthetic genes. Oring Life Evol Biosph. 1998;28:555–570. doi: 10.1023/A:1006531526299. [DOI] [PubMed] [Google Scholar]
- 58.Hood DW, Randle G, Cox AD, Makepeace K, Li J, Schweda EK, Richards JC, Moxon ER. Biosynthesis of cryptic lipopolysaccharide glycoforms in Haemophilus influenzae involves a mechanism similar to that required for O-antigen synthesis. J Bacteriol. 2004;186:7429–7439. doi: 10.1128/JB.186.21.7429-7439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones PA, Samuels NM, Phillips NJ, Munson RS, Jr, Bozue JA, Arseneau JA, Nichols WA, Zaleski A, Gibson BW, Apicella MA. Haemophilus influenzae type b strain A2 has multiple sialyltransferases involved in lipooligosaccharide sialylation. J Biol Chem. 2002;277:14598–14611. doi: 10.1074/jbc.M110986200. [DOI] [PubMed] [Google Scholar]
- 60.Tirado-Lee L, Lee A, Rees DC, Pinkett HW. Classification of a Haemophilus influenzae ABC transporter HI1470/71 through its cognate molybdate periplasmic binding protein, MolA. Structure. 2011;19:1701–1710. doi: 10.1016/j.str.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masters SL, Howlett GJ, Pau RN. The molybdate binding protein Mop from Haemophilus influenzae–biochemical and thermodynamic characterisation. Arch Biochem Biophys. 2005;439:105–112. doi: 10.1016/j.abb.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 62.Norskov-Lauritsen N, Bruun B, Kilian M. Multilocus sequence phylogenetic study of the genus Haemophilus with description of Haemophilus pittmaniae sp. nov. Int J Syst Evol Microbiol. 2005;55:449–456. doi: 10.1099/ijs.0.63325-0. [DOI] [PubMed] [Google Scholar]
- 63.Hedegaard J, Okkels H, Bruun B, Kilian M, Mortensen KK, Norskov-Lauritsen N. Phylogeny of the genus Haemophilus as determined by comparison of partial infB sequences. Microbiology. 2001;147:2599–2609. doi: 10.1099/00221287-147-9-2599. [DOI] [PubMed] [Google Scholar]
- 64.Bruun B, Gahrn-Hansen B, Westh H, Kilian M. Clonal relationship of recent invasive Haemophilus influenzae serotype f isolates from Denmark and the United States. J Med Microbiol. 2004;53:1161–1165. doi: 10.1099/jmm.0.45749-0. [DOI] [PubMed] [Google Scholar]
- 65.Wong SM, Akerley BJ. Genome-scale approaches to identify genes essential for Haemophilus influenzae pathogenesis. Front Cell Infect Microbiol. 2012;2:23. doi: 10.3389/fcimb.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharpe SW, Kuehn MJ, Mason KM. Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of nontypeable Haemophilus influenzae. Infect Immun. 2011;79:4361–4369. doi: 10.1128/IAI.05332-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Ulsen P, van Schilfgaarde M, Dankert J, Jansen H, van Alphen L. Genes of non-typeable Haemophilus influenzae expressed during interaction with human epithelial cell lines. Mol Microbiol. 2002;45:485–500. doi: 10.1046/j.1365-2958.2002.03025.x. [DOI] [PubMed] [Google Scholar]
- 68.Edwards JS, Palsson BO. Systems properties of the Haemophilus influenzae Rd metabolic genotype. J Biol Chem. 1999;274:17410–17416. doi: 10.1074/jbc.274.25.17410. [DOI] [PubMed] [Google Scholar]
- 69.Tatusov RL, Mushegian AR, Bork P, Brown NP, Hayes WS, Borodovsky M, Rudd KE, Koonin EV. Metabolism and evolution of Haemophilus influenzae deduced from a whole-genome comparison with Escherichia coli. Curr Biol. 1996;6:279–291. doi: 10.1016/S0960-9822(02)00478-5. [DOI] [PubMed] [Google Scholar]
- 70.Mell JC, Shumilina S, Hall IM, Redfield RJ. Transformation of natural genetic variation into Haemophilus influenzae genomes. PLoS Pathog. 2011;7:e1002151. doi: 10.1371/journal.ppat.1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin K, Morlin G, Smith A, Nordyke A, Eisenstark A, Golomb M. The tryptophanase gene cluster of Haemophilus influenzae type b: evidence for horizontal gene transfer. J Bacteriol. 1998;180:107–118. doi: 10.1128/jb.180.1.107-118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sondergaard A, San Millan A, Santos-Lopez A, Nielsen SM, Gonzalez-Zorn B, Norskov-Lauritsen N. Molecular organization of small plasmids bearing blaTEM-1 and conferring resistance to beta-lactams in Haemophilus influenzae. Antimicrob Agents Chemother. 2012;56:4958–4960. doi: 10.1128/AAC.00408-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams BJ, Golomb M, Phillips T, Brownlee J, Olson MV, Smith AL. Bacteriophage HP2 of Haemophilus influenzae. J Bacteriol. 2002;184:6893–6905. doi: 10.1128/JB.184.24.6893-6905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Power PM, Bentley SD, Parkhill J, Moxon ER, Hood DW. Investigations into genome diversity of Haemophilus influenzae using whole genome sequencing of clinical isolates and laboratory transformants. BMC Microbiol. 2012;12:273. doi: 10.1186/1471-2180-12-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Corn PG, Anders J, Takala AK, Kayhty H, Hoiseth SK. Genes involved in Haemophilus influenzae type b capsule expression are frequently amplified. J Infect Dis. 1993;167:356–364. doi: 10.1093/infdis/167.2.356. [DOI] [PubMed] [Google Scholar]
- 76.Kroll JS, Moxon ER. Capsulation and gene copy number at the cap locus of Haemophilus influenzae type b. J Bacteriol. 1988;170:859–864. doi: 10.1128/jb.170.2.859-864.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Papazisi L, Ratnayake S, Remortel BG, Bock GR, Liang W, Saeed AI, Liu J, Fleischmann RD, Kilian M, Peterson SN. Tracing phylogenomic events leading to diversity of Haemophilus influenzae and the emergence of Brazilian Purpuric Fever (BPF)-associated clones. Genomics. 2010;96:290–302. doi: 10.1016/j.ygeno.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moran GP, Coleman DC, Sullivan DJ. Comparative genomics and the evolution of pathogenicity in human pathogenic fungi. Eukaryot Cell. 2011;10:34–42. doi: 10.1128/EC.00242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilsdorf JR, Marrs CF, Foxman B. Haemophilus influenzae: genetic variability and natural selection to identify virulence factors. Infect Immun. 2004;72:2457–2461. doi: 10.1128/IAI.72.5.2457-2461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raffel FK, Szelestey BR, Beatty WL, Mason KM. The Haemophilus influenzae Sap transporter mediates bacterium-epithelial cell homeostasis. Infect Immun. 2013;81:43–54. doi: 10.1128/IAI.00942-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wyckoff EE, Schmitt M, Wilks A, Payne SM. HutZ is required for efficient heme utilization in Vibrio cholerae. J Bacteriol. 2004;186:4142–4151. doi: 10.1128/JB.186.13.4142-4151.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Champion MD, Zeng Q, Nix EB, Nano FE, Keim P, Kodira CD, Borowsky M, Young S, Koehrsen M, Engels R, et al. Comparative genomic characterization of Francisella tularensis strains belonging to low and high virulence subspecies. PLoS Pathog. 2009;5:e1000459. doi: 10.1371/journal.ppat.1000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merhej V, Georgiades K, Raoult D. Postgenomic analysis of bacterial pathogens repertoire reveals genome reduction rather than virulence factors. Brief Funct Genomics. 2013;12:291–304. doi: 10.1093/bfgp/elt015. [DOI] [PubMed] [Google Scholar]
- 84.Bliven KA, Maurelli AT. Antivirulence genes: insights into pathogen evolution through gene loss. Infect Immun. 2012;80:4061–4070. doi: 10.1128/IAI.00740-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 86.Fraser CM, Gocayne JD, White O, Adams MD, Clayton RA, Fleischmann RD, Bult CJ, Kerlavage AR, Sutton G, Kelley JM, et al. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 87.Steelman SM, Hein TW, Gorman A, Bix GJ. Effects of histidine-rich glycoprotein on cerebral blood vessels. J Cereb Blood Flow Metab. 2013;33:1373–1375. doi: 10.1038/jcbfm.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kopple JD, Swendseid ME. Effect of histidine intake of plasma and urine histidine levels, nitrogen balance and N tau-methylhistidine excretion in normal and chronically uremic men. J Nutr. 1981;111:931–942. doi: 10.1093/jn/111.6.931. [DOI] [PubMed] [Google Scholar]
- 89.Block WD, Westhoff MH, Steele BF. Histidine metabolism in the human adult: histidine blood tolerance, and the effect of continued free L-histidine ingestion on the concentration of imidazole compounds in blood and urine. J Nutr. 1967;91:189–194. doi: 10.1093/jn/91.2.189. [DOI] [PubMed] [Google Scholar]
- 90.Allaway WH, Kubota J, Losee F, Roth M. Selenium, molybdenum, and vanadium in human blood. Arch Environ Health. 1968;16:342–348. doi: 10.1080/00039896.1968.10665069. [DOI] [PubMed] [Google Scholar]
- 91.Greiner LL, Watanabe H, Phillips NJ, Shao J, Morgan A, Zaleski A, Gibson BW, Apicella MA. Nontypeable Haemophilus influenzae strain 2019 produces a biofilm containing N-acetylneuraminic acid that may mimic sialylated O-linked glycans. Infect Immun. 2004;72:4249–4260. doi: 10.1128/IAI.72.7.4249-4260.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Langereis JD, Hermans PW. Novel concepts in nontypeable Haemophilus influenzae biofilm formation. FEMS Microbiol Lett. 2013;346:81–89. doi: 10.1111/1574-6968.12203. [DOI] [PubMed] [Google Scholar]
- 93.Lappann M, Vogel U. Biofilm formation by the human pathogen Neisseria meningitidis. Med Microbiol Immunol. 2010;199:173–183. doi: 10.1007/s00430-010-0149-y. [DOI] [PubMed] [Google Scholar]
- 94.Ulanova M, Tsang RS. Haemophilus influenzae serotype a as a cause of serious invasive infections. Lancet Infect Dis. 2014;14:70–82. doi: 10.1016/S1473-3099(13)70170-1. [DOI] [PubMed] [Google Scholar]
- 95.Bruce MG, Zulz T, DeByle C, Singleton R, Hurlburt D, Bruden D, Rudolph K, Hennessy T, Klejka J, Wenger JD. Haemophilus influenzae serotype a invasive disease, Alaska, USA, 1983–2011. Emerg Infect Dis. 2013;19:932–937. doi: 10.3201/eid1906.121805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Redfield RJ, Findlay WA, Bosse J, Kroll JS, Cameron AD, Nash JH. Evolution of competence and DNA uptake specificity in the Pasteurellaceae. BMC Evol Biol. 2006;6:82. doi: 10.1186/1471-2148-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: List of primers and PCR conditions used in present study. (PDF 13 KB)
Additional file 2: ACT view of multiple genome alignment among human-related Haemophilus spp. Respective genome designations are indicated on the right hand side of each genome line. Forward (+) and complement (−) strands of individual genomes are indicated in the grey genome lines. Genomes are shown in full length and drawn to scale. Direct and inverted synteny between individual ORF (not indicated here) of the compared genomes are shown in red and blue, respectively. The level of amino acid similarity is represented by color shading with ascending saturation and indicates higher similarity. (PDF 243 KB)
Additional file 3: List of loci encoded at RgD F s of the Hif KR494 genome. (PDF 7 KB)
Additional file 4: Predicted genetic islands of serotype f (Gif KR494 ) defined in the Hif KR494 genome. (PDF 10 KB)
Additional file 5: Histidine auxotrophic and antibiotics susceptibility assay. (PDF 54 KB)
Additional file 6: Cross species genomic comparative of the closely-related human Haemophilus spp. A cross species genomic comparison of H. influenzae type f KR494 and human Haemophilus spp (H. aegyptius ATCC 11116, H. haemolyticus M21639 and H. parainfluenzae ATCC 33392). COG distribution and functionality classification of Hif KR494 CDSs that is (A) commonly shared and (B) unique CDSs in regards to the related Haemophilus spp. (PDF 107 KB)
Additional file 7: Map of RgD F in the H. influenzae type f KR494 genome in relative to the closely-related human Haemophilus species. Circular representation of protein conservation between Hif KR494 and the reference species was visualized using DNA plotter. From the outside in, the outer circle shows the genome length of Hif KR494 with position markers. The second circle shows the total ORFs of KR494 genome predicted on both forward and reverse strands. Common and unique ORFs in relative to the reference species are colored in blue and magenta, respectively. Phage-related ORFs are marked in yellow and orange. The third to fifth circles represent the distribution of individual ORF with high homology (≥85% similarity) (in red) to the corresponding ORF in reference species H. aegyptius ATCC11116, H. haemolyticus M21639 and H. parainfluenzae ATCC33392, respectively. Gaps between the conserved ORFs represent RgDF between Hif KR494 and the compared species, and were denoted as RgDF1 to RgDF7 (marked with green lines). GC plot and GC skew of the Hif KR494 genome are shown in the sixth and seventh circle, respectively. The genome of Hif KR494 was less conserved with H. aegyptius, H. haemolyticus and H. parainfluenzae at the RgDF1, 2, 3, 5, 6 and 7. The RgDF7 comprises xylFGH and xylAB operons (HifGL_000770-HifGL_000777) that are involved in xylose uptake and metabolism through the pentose phosphate pathway. This indicated that H. aegyptius, H. haemolyticus and H. parainfluenzae lacked a xylose metabolism system that is, however, conserved in H. influenzae. (PDF 323 KB)
Additional file 8: Total genomic comparison of Hif KR494 with H. influenzae reference strains, H. aegyptius, H. haemolyticus and H. parainfluenzae. Unique genes (133 CDSs) of Hif KR494 that consistently lacked homology with any of the aligned species were delineated based on the COG database. Notably, when genes of unknown function were excluded, most of the universal unique CDSs of Hif KR494 were phage-related products, followed by extracellular structures. The data represent the universal gene feature of Hif KR494. (PDF 77 KB)


