Figure 1.
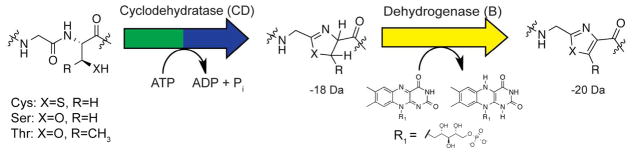
Synthesis of thiazole and oxazole heterocycles occurs over two distinct steps. First, the cyclodehydratase (C- and D- proteins) cyclizes cysteine, serine, or threonine residue into a thiazoline or oxazoline heterocycle through an ATP-dependent mechanism. Subsequently, a FMN-dependent dehydrogenase (B-protein) catalyzes the 2-electron oxidation to the azole heterocycle, resulting in a 20 Da mass loss from the unmodified amino acid.
