Figure 3.
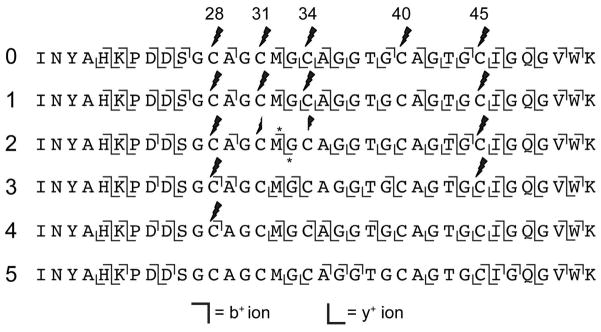
MS/MS fragmentation of BalhA1 azoline intermediates. BalhA1 reactions with BalhC/D to produce thiazoline-containing intermediates were treated with trypsin and iodoacetamide to identify unreacted cysteines. Subsequent addition of formic acid returned all thiazolines back to cysteine, at which point the samples were subjected to LC-FTMS/MS. The fragment ions observed for various ring intermediates are denoted by the b+ and y+ ion symbols. The leftmost numbers indicate ring state. Across the top, cysteines are numbered based on the sequence of full length BalhA1. Lightning bolts signify locations labeled with iodoacetamide. Asterisks denote ions containing a mixed population of two peptides.
