Abstract
Accumulating evidences indicate that some diseases are triggered by abnormalities of the gut microbiota. Among these, immune-related diseases can be the promising targets for probiotcs. Several studies have proved the efficacy of probiotics for preventing such diseases including cancers, infections, allergies, inflammatory bowel diseases and autoimmune diseases. Lactobacillus casei strain Shirota (LcS) is one of the most popular probiotics, benefits of which in health maintenance and disease control have been supported by several science-based evidences. This review summarizes human clinical trials with this probiotic against cancer development and also discusses the possible immunomodulatory mechanisms by which LcS exerts anti-cancer activity.
Keywords: Cancer prevention, immune modulation, interleukin (IL)-12, Lactobacillus casei Shirota (LcS), natural killer (NK) cell, probiotic
Introduction
Human intestine harbours 100 trillion bacteria with more than a thousand species, and the commensal gut microbiota forms a symbiotic relationship with the host1,2. Some of the gut bacteria provide benefits to the host by producing vitamins and short chain fatty acids, maintaining gut epithelial barrier and providing colonization resistance against pathogens3,4. More than half of immune competent cells in the body distribute into the intestine. The gut microbiota plays a pivotal role in the development of the immune system and continues to interact with intestinal immune cells to maintain or modulate their functions5,6. Probiotics, that are orally ingested as beneficial bacteria, can survive the intestinal tract, recover/maintain balanced gut microbiota, and then modulate immune functions7. Thus, these can be a promising tool to control some diseases caused by dysregulated immune responses.
Gut microbiota and cancer
Several factors, including unbalanced diet, ageing, mental stress, infections and antibiotic use, impair the balance of gut microbiota, and abnormalities in the gut microbiota can become the causes of some diseases. Correlation between altered gut microbiota and some of the immune related diseases, including inflammatory bowel diseases (IBD), allergy and obesity, has been demonstrated8,9,10.
The gut microbiota is considered to be closely related with the risk of cancer11. Thousands of abnormal cells with genetic mutations are generated every day in our body. Our immune surveillance system plays a critical role in clearance of such cells to prevent the development of cancer. Gut bacteria may affect the defense against cancer by modulating immune functions of the host. Some gut bacteria generate carcinogens and tumour-promoting substances including heterocyclic amines and secondary bile acids while others produce beneficial metabolites to prevent cancer, such as short chain fatty acids and equol12,13. Analysis of faecal bacterial composition showed an increase in the population of Bacteroides and Prevotella in colorectal cancer patients14. Increased Dorea spp. and Faecalibacterium spp. were observed in colonic mucosa-associated microbiota in patients with colorectal adenoma15. If probiotics promote the gut microbiota toward the well-balanced composition and maintain sufficient immunosurveillance, that would be valuable for cancer prevention.
Probiotics and their beneficial effects
Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”, and lactobacilli and bifidobacteria are currently the most popular probiotics7. They can survive in the intestine and promote recovery of normal gut microbiota. Therefore, several health benefits of probiotics, including control of gastroenteritis, irritable bowel syndrome, hepatic diseases and cardiovascular diseases, have been proposed. Among these, much attention has been paid to their immune regulatory activity16,17. Decreased immune defense functions tend to increase the risk of infections and cancers while excessively activated or dysregulated immune responses cause inflammatory bowel diseases, allergy and autoimmune diseases18. Probiotics are expected to regulate immune responses to prevent such diseases. Thus, these are often used both in clinical applications and as foods that maintain the immunologic homeostasis of the host.
Probiotics and cancer prevention
Although several animal experiments suggest the efficacy of probiotics for cancer prevention12,19, there have not been much information obtained from human clinical trials for such benefits. The SYNCAN project20, supported by the European Community, conducted a clinical trial to evaluate the effect of synbiotic (probiotic plus prebiotic) preparation on colorectal cancer. A double-blind, placebo-controlled randomized trial was performed in 37 colon cancer patients and 43 polypectomized patients. Intervention with a synbiotic composed of Lactobacillus rhamnosus GG, Bifidobacterium lactis Bb12 and oligofructose-enriched inulin for 12 wk resulted in favourable changes in the gut microbiota with increased lactobacilli and bifidobacteria and decreased Clostridium perfringens20. The intervention also reduced proliferation and DNA damage in colonic mucosa and the capacity of faecal water samples to induce necrosis in colonic cells in polypectomized patients. Increased production of interferon (IFN)-γ by peripheral blood mononuclear cells (PBMC) was observed in the cancer patients20. Thus, several colorectal biomarkers were shown to be improved by the synbiotic treatment. A prospective study on 45,241 subjects in the EPIC-Italy cohort suggested the preventive effect of probiotics on colorectal cancer21. This epidemiological study explained that high yogurt intake was significantly associated with decreased colorectal cancer risk. As for cases other than colorectal cancer, Lactobacillus casei strain Shirota (LcS) is the only probiotic strain for which clinical trials have supported the favourable effects22,23,24.
Clinical trials with LcS against cancer
LcS is one of the most popular probiotics and has been used in producing fermented milk drink for more than 75 years. LcS can survive in the intestine, modulates the gut microbiota and provides several benefits to the host25,26. In particular, the effects of LcS on the host immune system have been intensively investigated27,28.
Several animal studies have demonstrated that LcS augments the functions of macrophages, natural killer (NK) cells and T cells and exerts anti-cancer activity29,30,31. Aso et al22 conducted a multicenter, double-blind, placebo-controlled, randomized trial to evaluate the efficacy of LcS on the recurrence of superficial bladder cancer. One hundred and thirty eight patients with primary bladder tumours were allocated to the two groups after transurethral resection of tumours. They were orally given freeze-dried LcS preparation (3 × 1010 cfu/day) or placebo daily for one year. LcS ingestion significantly prevented the recurrence of bladder cancer (the corrected cumulative recurrence-free rate at one year: 79.2% in the LcS group; 54.9% in the placebo group, P=0.01).
Another multicenter, prospective, randomized, controlled trial was performed to evaluate whether oral LcS administration could enhance the efficacy of intravesical chemotherapy in patients who underwent transurethral resection for superficial bladder cancer23. Two hundred and seven patients were assigned to the two groups; one group was treated transurethrally with epirubicin for three months while another group was treated with daily oral administration of freeze-dried LcS (3 × 1010 cfu/day) for one year in addition to the epirubicin treatment. The three year recurrence-free rate was significantly higher in the epirubicin plus LcS group (74.6% in the epirubicin plus LcS group; 59.9% in the epirubicin group, P=0.02), although the overall survival did not differ between the groups23. A case-control study to assess the preventive effect of LcS intake against bladder cancer was conducted by Ohashi et al32. One hundred and eighty cases and 445 population-based controls were examined. The adjusted odds ratio of previous intake of LcS-containing fermented milk 1-2 times/week was 0.46, suggesting that habitual intake of LcS reduces the risk of bladder cancer. Taken together, all these results strongly support the protective role of LcS against bladder cancer.
The preventive effects of LcS on other cancers were tested by a large-scale randomized trial. Three hundred and ninety eight subjects in whom at least two colorectal tumours had been removed, participated in the study that was designed to evaluate effects of LcS and wheat bran24. The subjects were assigned to four groups administered daily freeze-dried LcS preparation (3 × 1010 cfu/day), wheat bran, both or neither, and the recurrence of colorectal tumours was observed for four years. LcS intake significantly reduced the recurrence rate of tumours with a grade of moderate atypia or higher (the adjusted odds ratio in LcS group: 0.80 at 2 years; 0.65 at 4 years), but wheat bran intake did not24. No difference in the development of new tumours was detected in either group. The results suggested that LcS prevented atypia of colorectal tumours.
All these intervention studies together with the epidemiological survey strongly suggest that LcS prevents the development of certain cancers in humans. Furthermore, Kanazawa et al33 demonstrated the benefit of LcS against postoperative infectious complications in biliary cancer patients undergoing high-risk hepatectomy. Forty four patients with biliary cancer were allocated to two groups. One group received post-operative enteral feeding that included LcS, Bifidobacterium breve strain Yakult (each 3 × 1010 cfu/day) and galacto-oligosaccharides (12 g/day) from postoperative day 1 to day 14, and the other group received enteral feeding only. The synbiotic treatment improved the balance of gut microbiota and reduced postoperative infections. The same group further confirmed that additional perioperative treatment with the same synbiotic was more effective for such patients in preventing postoperative infectious complications34. In this study, augmented NK cell activity was observed in PBMC after the perioperative synbiotic treatment.
Mechanisms of cancer prevention by LcS
Various mechanisms of the anti-cancer activity of LcS have been proposed from animal studies. Among these, augmentation/recovery of NK cell activity is reliable. Takagi et al35 examined the mechanism of anti-cancer action of LcS using mutant mice deficient in NK cell activity. In a 3-methylcholanthrene-induced cancer model, oral administration of LcS delayed the development of fibrosarcoma in wild-type mice while the anti-cancer activity of LcS was not observed in beige mice, which are congenitally defective in NK cell activity. Furthermore, LcS administration recovered the decreased NK cell activity in 3-methylcholanthrene-treated C3H/HeN mice35. These findings strongly suggest that LcS exerts its anti-cancer effect by augmenting NK cell activity.
Another anti-cancer mechanism of LcS through the modulation of host immune responses has also been suggested. It is now proposed that colorectal cancer may develop from severe colonic inflammation. In a mouse model of colitis-associated cancer, LcS inhibited interleukin (IL)-6-mediated inflammatory responses in the colonic mucosa and suppressed the development of cancer36. The results suggest that LcS can downregulate inflammation and then prevent the subsequent cancer development. Mechanisms other than immunomodulation are also proposed. Carcinogenic activity of metabolic substances generating in the intestine may be reduced by LcS intake. LcS ingestion may modulate the composition of gut microbiota and change their metabolic activity, resulting in a decrease of carcinogens such as heterocyclic amines and p-cresol37,38. LcS possibly adsorbs carcinogenic compounds to be excreted39. Thus, LcS is thought to reduce the risk of cancer development through various mechanisms (Table).
Table.
Proposed mechanisms of cancer prevention by Lactobacillus casei Shirota (LcS)

Recovery of NK cell activity by LcS
NK cells are crucial for immunosurveillance against cancers and infections, and higher NK cell activity has been reported to be associated with lower risk of cancer40. Since NK cell activity can be altered by various environmental factors including ageing, severe mental stress, smoking and chronic infections, maintaining NK cell activity within the suitable range is a promising target for probiotics. Several clinical trials have been conducted to examine whether LcS intake augments the NK cell activity in subjects with decreased NK cell activity.
A placebo-controlled, cross-over trial in elderly subjects (69˜97 yr old) was conducted. Ten subjects were given LcS-containing fermented milk (4 × 1010 cfu/day) or placebo daily for three weeks. NK cell activity in the PBMC after intake of the fermented milk was significantly higher than that after intake of placebo41.
In another study, the effect of LcS was evaluated in young healthy subjects (20˜40 yr old) with low NK cell activity. Nine subjects with low NK cell activity were enrolled and provided LcS-containing fermented milk (4 × 1010 cfu/day) or placebo daily for three weeks in a cross-over design. The fermented milk significantly enhanced NK cell activity during the intake period and even 3 wk after the end of the intake compared to the levels before the intake, but placebo did not significantly enhance NK cell activity42.
A double-blind, placebo-controlled, randomized trial was conducted to evaluate whether LcS intake recovers NK cell activity in habitual smokers. Ninety-nine smokers were assigned to two groups and given LcS-containing fermented milk (4 × 1010 cfu/day) or placebo daily for three weeks. NK cell activity was adjusted by the numbers of cigarettes smoked before analyzing LcS efficacy, since NK cell activity in individuals was inversely correlated to the number of cigarettes smoked in this study43. The results explained that the adjusted NK cell activity in the LcS group was significantly higher than the placebo group.
A prospective, uncontrolled trial in patients with human T lymphotropic virus-1-associated myelopathy (HAM) was conducted. HAM is a neurological disorder driven by the chronic infection with the causal virus of adult T cell leukemia, and NK cell activity in the patients is lower than the healthy level. Ten HAM patients took LcS-containing fermented milk (8 × 1010 cfu/day) daily for four weeks. Intake of the fermented milk significantly enhanced NK cell activity and improved the neurological symptoms of the patients44.
Mechanism of augmenting NK cell activity
We examined the possible mechanisms of LcS to augment NK cell activity using cultures of human PBMC. Addition of LcS into the cultures of PBMC enhanced NK cell activity45. The ability of LcS to augment NK cell activity was greater than those of other probiotic strains tested, and the ability to augment NK cell activity seemed to correlate with the ability of the strains to induce IL-12 production. LcS failed to augment NK cell activity when monocytes were depleted from PBMC. We further revealed that LcS stimulated monocytes to secrete IL-12, and addition of anti-IL-12 antibody to the cultures reduced the ability of LcS to augment NK cell activity41,45. Taken together, all these results suggest that LcS activates NK cells through the induction of IL-12 production by monocytes/macrophages. Mechanisms other than IL-12 production may be involved, since neutralization of IL-12 did not completely stop the LcS-derived augmentation of NK cell activity.
Though it has been demonstrated that monocytes were essential for LcS to augment NK cell activity during in vitro stimulation of PBMC, macrophages and dendritic cells in the intestine are likely to be the first targets for orally ingested LcS. To understand the mechanisms of probiotic action of LcS more clearly, in vivo studies focusing on the responses of mucosal immune cells are now underway in our laboratory.
Induction of IL-12 production by LcS
Using mouse peritoneal macrophages, we have revealed how LcS can induce a large amount of IL-12, a key cytokine in augmenting host immune defense including NK cell activity. LcS has the specific cell wall composed of peptidoglycan which is covered by two types of uncharged polysaccharides, PS-1 and PS-246. The cell wall of LcS is highly resistant to a digesting enzyme, N-acetylmuramidase, probably because the densely associated polysaccharides may hinder the access of the enzyme to its cleavage sites in the peptidoglycan47,48. Studies with mouse peritoneal macrophages demonstrated that phagocytosis of LcS is essential for its induction of IL-12 production by macrophages, but that recognition of LcS by Toll-like receptor (TLR)2, a sensor for a variety of Gram-positive bacteria, is not required for IL-12 induction47. The active component of LcS for the IL-12 induction has been revealed to be the intact cell wall, and the LcS bacterial body engulfed by the macrophages showed significant resistance to digestion (Figure). These results indicate that the rigid cell wall of LcS that is resistant to intracellular digestion, effectively stimulates macrophages to secrete a large amount of IL-12. Our next question is to unveil how the tertiary structure of the LcS intact cell wall is recognized by the phagocytes to produce IL-12.
Fig.
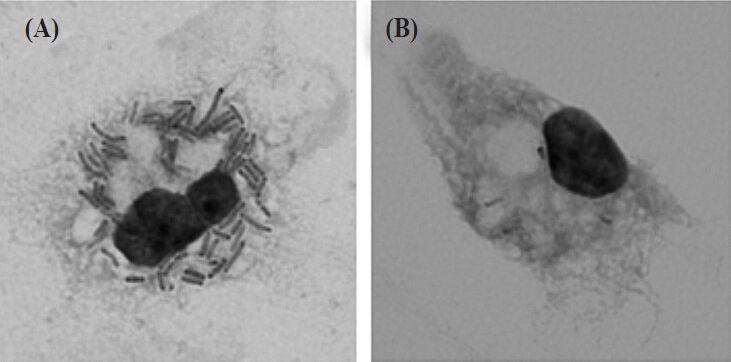
Resistance of Lactobacillus casei Shirota (LcS) to intracellular digestion in macrophages. Mouse peritoneal macrophages were cultured with LcS (A) and L. johnsonii JCM 2012T (B) for 24 h and stained with Giemsa. Lysis of lactobacilli in macrophages was observed by light microscopy. LcS engulfed by macrophages (arrows) shows significant resistance to digestion. Original magnification: × 1000.
Concluding remarks
In summary, LcS can augment host immune defense through induction of IL-12 production by phagocytes, in which the unique cell wall structure of this probiotic organism plays a key role. The augmented immune functions would be helpful for cancer prevention. Accumulating evidence obtained from human clinical trials with LcS supports the idea that specific probiotics can be applicable for prevention or control of cancers.
Human clinical trials in patients with allergy or ulcerative colitis and studies in animal models for autoimmune diseases showed that LcS intake did not exhibit any harmful effects and rather ameliorated clinical symptoms and disease biomarkers49,50,51,52,53,54. The mechanisms of action of LcS have been thought to be in two ways, augmentation of immune defense functions in the immunocompromised state and downregulation of excessive immune responses in the inflammatory state, but remain to be elucidated. Recent findings explain that LcS, which is a potent IL-12 inducer, can also work as a potent inducer of immunosuppressive IL-10, depending on the conditions of phagocytes that it stimulates55. The flexible cytokine production induced by LcS may be a possible mechanism by which the probiotic exerts multifunctional immune regulatory activities18. In order to fully understand the sophisticated actions of probiotics, future studies should be performed taking into consideration the indirect control of immune responses by probiotics through modulation of the nervous and endocrine systems in addition to the direct actions on the immune system.
Acknowledgment
The authors thank to Dr Masanobu Nanno (Yakult Central Institute) for his valuable advice.
References
- 1.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–93. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–9. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–5. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 6.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–23. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microb Rev. 2003;16:658–72. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damaskos D, Kolios G. Probiotics and prebiotics in inflammatory bowel disease: microflora ‘on the scope’. Br J Clin Pharmacol. 2008;65:453–67. doi: 10.1111/j.1365-2125.2008.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62:1223–36. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Michelle Luo T, Jobin C, Young HA. Gut microbiota and probiotics in colon tumorigenesis. Cancer Lett. 2011;309:119–27. doi: 10.1016/j.canlet.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azcarate-Peril MA, Sikes M, Bruno-Barcena JM. The intestinal microbiota, gastrointestinal environment and colorectal cancer: a putative role for probiotics in prevention of colorectal cancer? Am J Physiol Gastrointest Liver Physiol. 2011;301:G401–24. doi: 10.1152/ajpgi.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akaza H. Prostate cancer chemoprevention by soy isoflavones: role of intestinal bacteria as the “second human genome”. Cancer Sci. 2012;103:969–75. doi: 10.1111/j.1349-7006.2012.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One. 2011;6:e16393. doi: 10.1371/journal.pone.0016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen XJ, Rawls JF, Randall T, Burcal L, Mpande CN, Jenkins N, et al. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes. 2010;1:138–47. doi: 10.4161/gmic.1.3.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shida K, Nanno M. Probiotics and immunology: separating the wheat from the chaff. Trends Immunol. 2008;29:565–73. doi: 10.1016/j.it.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 17.O’Flaherty S, Saulnier DM, Pot B, Versalovic J. How can probiotics and prebiotics impact mucosal immunity? Gut Microbes. 2010;1:293–300. doi: 10.4161/gmic.1.5.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shida K, Nanno M, Nagata S. Flexible cytokine production by macrophages and T cells in response to probiotic bacteria: a possible mechanism by which probiotics exert multifunctional immune regulatory activities. Gut Microbes. 2011;2:109–14. doi: 10.4161/gmic.2.2.15661. [DOI] [PubMed] [Google Scholar]
- 19.Rafter J. Lactic acid bacteria and cancer: mechanistic perspective. Br J Nutr. 2002;88(Suppl 1):S89–94. doi: 10.1079/BJN2002633. [DOI] [PubMed] [Google Scholar]
- 20.Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85:488–96. doi: 10.1093/ajcn/85.2.488. [DOI] [PubMed] [Google Scholar]
- 21.Pala V, Sieri S, Berrino F, Vineis P, Sacerdote C, Palli D, et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int J Cancer. 2011;129:2712–9. doi: 10.1002/ijc.26193. [DOI] [PubMed] [Google Scholar]
- 22.Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur Urol. 1995;27:104–9. doi: 10.1159/000475138. [DOI] [PubMed] [Google Scholar]
- 23.Naito S, Koga H, Yamaguchi A, Fujimoto N, Hasui Y, Kuramoto H, et al. Prevention of recurrence with epirubicin and Lactobacillus casei after transurethral resection of bladder cancer. J Urol. 2008;179:485–90. doi: 10.1016/j.juro.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa H, Akedo I, Otani T, Suzuki T, Nakamura T, Takeyama I, et al. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int J Cancer. 2005;116:762–7. doi: 10.1002/ijc.21115. [DOI] [PubMed] [Google Scholar]
- 25.Yuki N, Watanabe K, Mike A, Tagami Y, Tanaka R, Ohwaki M, et al. Survival of a probiotic, Lactobacillus casei strain Shirota, in the gastrointestinal tract: selective isolation from faeces and identification using monoclonal antibodies. Int J Food Microbiol. 1999;48:51–7. doi: 10.1016/s0168-1605(99)00029-x. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto K, Takada T, Shimizu K, Moriyama K, Kawakami K, Hirano K, et al. Effects of a probiotic fermented milk beverage containing Lactobacillus casei strain Shirota on defecation frequency, intestinal microbiota, and the intestinal environment of healthy individuals with soft stools. J Biosci Bioeng. 2010;110:547–52. doi: 10.1016/j.jbiosc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Nanno M, Kato I, Kobayashi T, Shida K. Biological effects of probiotics: what impact does Lactobacillus casei shirota have on us? Int J Immunopathol Pharmacol. 2011;24:45S–50S. [PubMed] [Google Scholar]
- 28.Nomoto K. Immunoregulatory functions of probiotics. Biosci Microflora. 2000;19:1–8. [Google Scholar]
- 29.Kato I, Kobayashi S, Yokokura T, Mutai M. Antitumor activity of Lactobacillus casei in mice. Gann. 1981;72:517–23. [PubMed] [Google Scholar]
- 30.Kato I, Yokokura T, Mutai M. Augmentation of mouse natural killer cell activity by Lactobacillus casei and its surface antigens. Microbiol Immunol. 1984;28:209–17. doi: 10.1111/j.1348-0421.1984.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 31.Kato I, Yokokura T, Mutai M. Correlation between increase in Ia-bearing macrophages and induction of T cell-dependent antitumor activity by Lactobacillus casei in mice. Cancer Immunol Immunother. 1988;26:215–21. doi: 10.1007/BF00199932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohashi Y, Nakai S, Tsukamoto T, Masumori N, Akaza H, Miyanaga N, et al. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int. 2002;68:273–80. doi: 10.1159/000058450. [DOI] [PubMed] [Google Scholar]
- 33.Kanazawa H, Nagino M, Kamiya S, Komatsu S, Mayumi T, Takagi K, et al. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg. 2005;390:104–13. doi: 10.1007/s00423-004-0536-1. [DOI] [PubMed] [Google Scholar]
- 34.Sugawara G, Nagino M, Nishio H, Ebata T, Takagi K, Asahara T, et al. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg. 2006;244:706–14. doi: 10.1097/01.sla.0000219039.20924.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis. 2001;22:599–605. doi: 10.1093/carcin/22.4.599. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto S, Hara T, Nagaoka M, Mike A, Mitsuyama K, Sako T, et al. A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology. 2009;128:e170–80. doi: 10.1111/j.1365-2567.2008.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Preter V, Geboes K, Verbrugghe K, De Vuyst L, Vanhoutte T, Huys G, et al. The in vivo use of the stable isotope-labelled biomarkers lactose-[15N]ureide and [2H4]tyrosine to assess the effects of pro- and prebiotics on the intestinal flora of healthy human volunteers. Br J Nutr. 2004;92:439–46. doi: 10.1079/bjn20041228. [DOI] [PubMed] [Google Scholar]
- 38.Hayatsu H, Hayatsu T. Suppressing effect of Lactobacillus casei administration on the urinary mutagenicity arising from ingestion of fried ground beef in the human. Cancer Lett. 1993;73:173–9. doi: 10.1016/0304-3835(93)90261-7. [DOI] [PubMed] [Google Scholar]
- 39.Morotomi M, Mutai M. In vitro binding of potent mutagenic pyrolysates to intestinal bacteria. J Natl Cancer Inst. 1986;77:195–201. [PubMed] [Google Scholar]
- 40.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–9. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 41.Takeda K, Suzuki T, Shimada SI, Shida K, Nanno M, Okumura K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clin Exp Immunol. 2006;146:109–15. doi: 10.1111/j.1365-2249.2006.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagao F, Nakayama M, Muto T, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Biosci Biotechnol Biochem. 2000;64:2706–8. doi: 10.1271/bbb.64.2706. [DOI] [PubMed] [Google Scholar]
- 43.Morimoto K, Takeshita T, Nanno M, Tokudome S, Nakayama K. Modulation of natural killer cell activity by supplementation of fermented milk containing Lactobacillus casei in habitual smokers. Prev Med. 2005;40:589–94. doi: 10.1016/j.ypmed.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Matsuzaki T, Saito M, Usuku K, Nose H, Izumo S, Arimura K, et al. A prospective uncontrolled trial of fermented milk drink containing viable Lactobacillus casei strain Shirota in the treatment of HTLV-1 associated myelopathy/tropical spastic paraparesis. J Neurol Sci. 2005;237:75–81. doi: 10.1016/j.jns.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Shida K, Suzuki T, Kiyoshima-Shibata J, Shimada S, Nanno M. Essential roles of monocytes in stimulating human peripheral blood mononuclear cells with Lactobacillus casei to produce cytokines and augment natural killer cell activity. Clin Vaccine Immunol. 2006;13:997–1003. doi: 10.1128/CVI.00076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagaoka M, Muto M, Nomoto K, Matuzaki T, Watanabe T, Yokokura T. Structure of polysaccharide-peptidoglycan complex from the cell wall of Lactobacillus casei YIT9018. J Biochem. 1990;108:568–71. doi: 10.1093/oxfordjournals.jbchem.a123243. [DOI] [PubMed] [Google Scholar]
- 47.Shida K, Kiyoshima-Shibata J, Nagaoka M, Watanabe K, Nanno M. Induction of interleukin-12 by Lactobacillus strains having a rigid cell wall resistant to intracellular digestion. J Dairy Sci. 2006;89:3306–17. doi: 10.3168/jds.S0022-0302(06)72367-0. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Rimpilainen M, Simelyte E, Toivanen P. Characterisation of Eubacterium cell wall: peptidoglycan structure determines arthritogenicity. Ann Rheum Dis. 2001;60:269–74. doi: 10.1136/ard.60.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamura M, Shikina T, Morihana T, Hayama M, Kajimoto O, Sakamoto A, et al. Effects of probiotics on allergic rhinitis induced by Japanese cedar pollen: randomized double-blind, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2007;143:75–82. doi: 10.1159/000098318. [DOI] [PubMed] [Google Scholar]
- 50.Mitsuyama K, Matsumoto S, Yamasaki H, Masuda J, Kuwaki K, Takedatsu H, et al. Beneficial effects of Lactobacilluc casei in ulcerative colitis: a pilot study. J Clin Biochem Nutr. 2008;43:s78–81. [Google Scholar]
- 51.Kato I, Endo-Tanaka K, Yokokura T. Suppressive effects of the oral administration of Lactobacillus casei on type II collagen-induced arthritis in DBA/1 mice. Life Sci. 1998;63:635–44. doi: 10.1016/s0024-3205(98)00315-4. [DOI] [PubMed] [Google Scholar]
- 52.Matsuzaki T, Nagata Y, Kado S, Uchida K, Kato I, Hashimoto S, et al. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of Lactobacillus casei. APMIS. 1997;105:643–9. doi: 10.1111/j.1699-0463.1997.tb05066.x. [DOI] [PubMed] [Google Scholar]
- 53.Mike A, Nagaoka N, Tagami Y, Miyashita M, Shimada S, Uchida K, et al. Prevention of B220+ T cell expansion and prolongation of lifespan induced by Lactobacillus casei in MRL/lpr mice. Clin Exp Immunol. 1999;117:368–75. doi: 10.1046/j.1365-2249.1999.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi T, Suzuki T, Kaji R, Serata M, Nagata T, Ando M, et al. Probiotic upregulation of peripheral IL-17 responses does not exacerbate neurological symptoms in experimental autoimmune encephalomyelitis mouse models. Immunopharmacol Immunotoxicol. 2012;34:423–33. doi: 10.3109/08923973.2010.617755. [DOI] [PubMed] [Google Scholar]
- 55.Kaji R, Kiyoshima-Shibata J, Nagaoka M, Nanno M, Shida K. Bacterial teichoic acids reverse predominant IL-12 production induced by certain Lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J Immunol. 2010;184:3505–13. doi: 10.4049/jimmunol.0901569. [DOI] [PubMed] [Google Scholar]


