Abstract
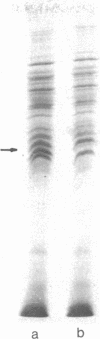
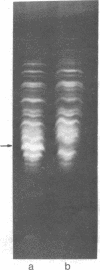
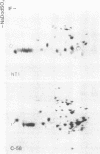
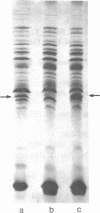
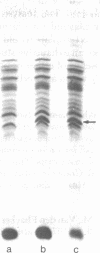
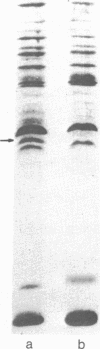
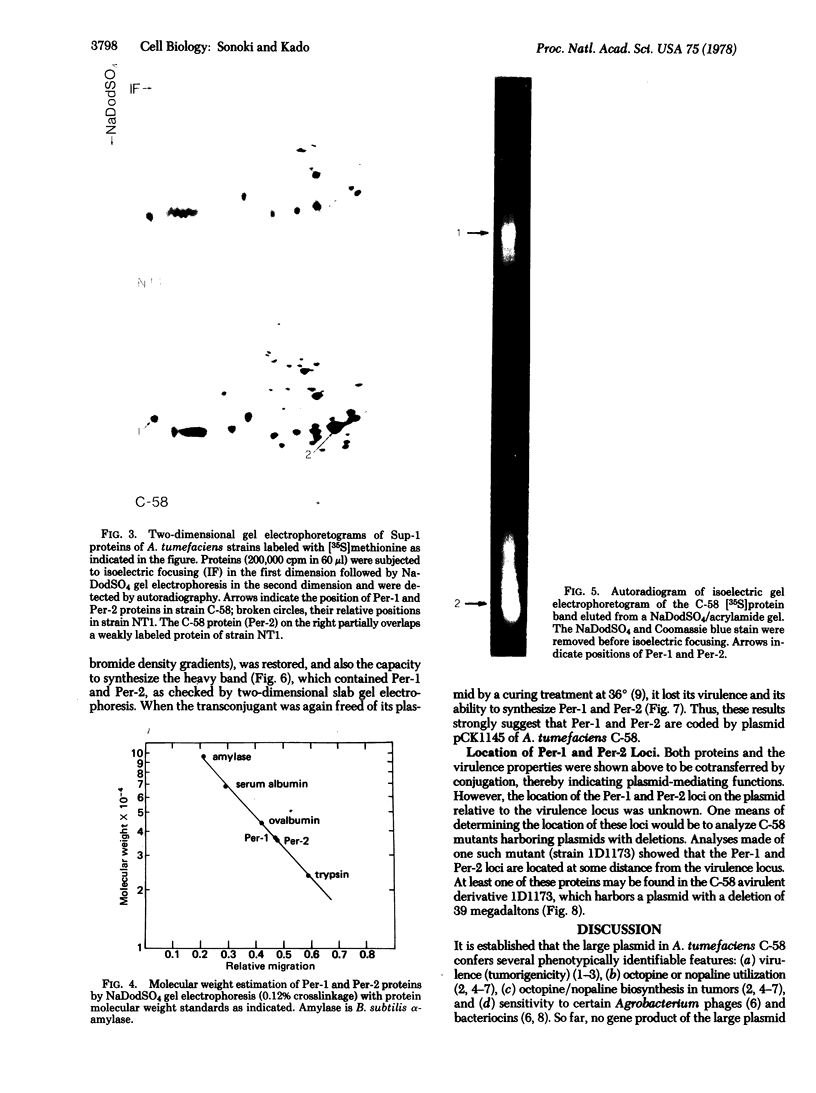
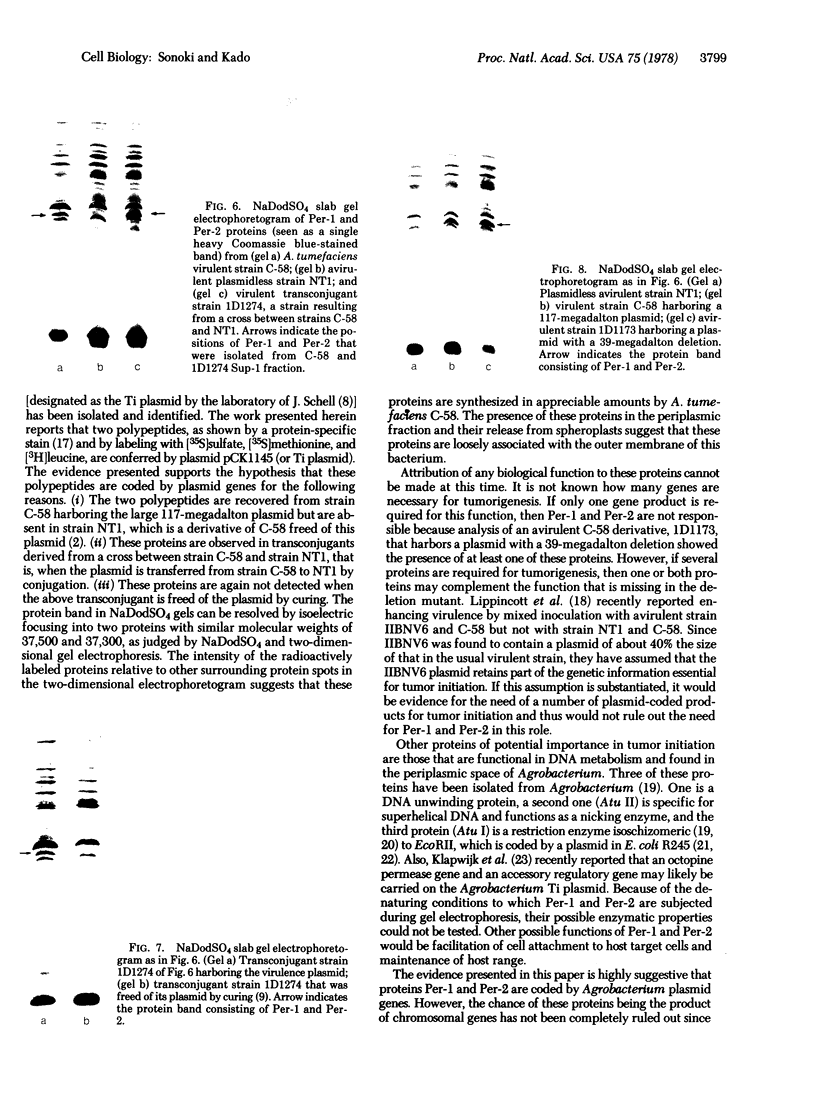
Membrane-associated and periplasmic proteins of Agrobacterium tumefaciens C-58 were compared with those from avirulent (nontumrigenic) derivative strains by slab and two-dimensional gel electrophoresis. Two proteins (Per-I and Per-2), with a molecular weight of 37,500 and 37,300, respectively, were detected in the supernatant fraction of cells of strain C-58 treated with EDTA and lysozyme in which a 117-megadalton plasmid confers virulence on the organism. The same proteins are missing in an avirulent plasmid-free derivative of C-58. When this derivative is mated with C-58, the resulting transconjugants regain the large C-58 plasmid together with the restoration of virulence and the expression of Per-1 and Per-2 proteins. When the transconjugants were cured of their plasmid, they concomitantly lost their virulence and Per-1 and Per-2. The functional roles of these proteins are unknown, but they are associated with the outer membrane and periplasmic fraction of the Agrobacterium cell. If directly involved in tumorigenesis, these proteins are not the sole determinants of tumorigenicity because they are synthesized in an avirulent derivative of C-58 that carries a deletion in the plasmid in the region conferring the tumorigenic phenotype. These results strongly suggest that the Per-1 and Per-2 proteins are plasmid-coded gene products. The possible roles of these proteins in specifying host range and host-cell attachment are also discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigger C. H., Murray K., Murray N. E. Recognition sequence of a restriction enzyme. Nat New Biol. 1973 Jul 4;244(131):7–10. doi: 10.1038/newbio244007a0. [DOI] [PubMed] [Google Scholar]
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Chow L. T., Dugaiczyk A., Hedgpeth J., Goodman H. M. DNA substrate site for the EcoRII restriction endonuclease and modification methylase. Nat New Biol. 1973 Jul 11;244(132):40–43. doi: 10.1038/newbio244040a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Engler G., Holsters M., Van Montagu M., Schell J., Hernalsteens J. P., Schilperoort Agrocin 84 sensitivity: a plasmid determined property in Agrobacterium tumefaciens. Mol Gen Genet. 1975 Jul 10;138(4):345–349. doi: 10.1007/BF00264804. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin B. C., Kado C. I. Studies on Agrobacterium tumefaciens. VIII. Avirulence induced by temperature and ethidium bromide. Can J Microbiol. 1977 Nov;23(11):1554–1561. doi: 10.1139/m77-229. [DOI] [PubMed] [Google Scholar]
- Lippincott B. B., Margot J. B., Lippincott J. A. Plasmid content and tumor initiation complementation by Agrobacterium tumefaciens IIBNV6. J Bacteriol. 1977 Dec;132(3):824–831. doi: 10.1128/jb.132.3.824-831.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation and properties of outer and cytoplasmic membranes in Escherichia coli. Biochim Biophys Acta. 1969;193(2):268–276. doi: 10.1016/0005-2736(69)90188-6. [DOI] [PubMed] [Google Scholar]
- Montoya A. L., Chilton M. D., Gordon M. P., Sciaky D., Nester E. W. Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J Bacteriol. 1977 Jan;129(1):101–107. doi: 10.1128/jb.129.1.101-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Roizès G., Patillon M., Kovoor A. A restriction endonuclease from Agrobacterium tumefaciens. FEBS Lett. 1977 Oct 1;82(1):69–70. doi: 10.1016/0014-5793(77)80887-9. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N., Engler G., Holsters M., Van den Elsacker S., Zaenen I., Schilperoort R. A., Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974 Nov 8;252(5479):169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N., Genetello C., Schell J., Schilperoort R. A., Hermans A. K., Van Montagu M., Hernalsteens J. P. Acquisition of tumour-inducing ability by non-oncogenic agrobacteria as a result of plasmid transfer. Nature. 1975 Jun 26;255(5511):742–743. doi: 10.1038/255742a0. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]