Abstract
Aim:
The aim of this study is to evaluate the role of 68Ga-DOTATATE positron emission tomography/computed tomography (PET/CT) scan for the detection of bone metastases in pediatric neuroendocrine tumors (NETs) and to compare it with CT scan.
Materials and Methods:
A total of 30 patients (18 were males and 12 were females; age range: 1-18 years; mean age 7.6 years) with histologically confirmed NETs referred to our department were retrospectively analyzed. All patients underwent 68Ga-DOTATATE PET/CT scan at the time of diagnosis for primary staging. Contrast enhanced CT (CECT) performed at the time of PET scan acquisition was used for comparison with PET data. Imaging results were analyzed on a per-patient and on a per-lesion basis. Clinical follow-up of all patients and repeat PET/CT imaging (n = 10) was taken as the reference standard.
Results:
Out of the 30 patients, 17 had no evidence of bone metastases on any imaging modality or on clinical follow-up while the rest of 13 patients showed evidence of bone metastases (nine showing positivity both on 68Ga-DOTATATE PET and CT scan while four showing positivity only on 68Ga-DOTATATE PET). Compared with CT scan, 68Ga-DOTATATE PET detected bone metastases at a significantly higher rate (P = 0.0039). On a per lesion analysis, out of a total of 225 lesions detected by 68Ga-DOTATATE PET, only 84 lesions could be detected by CT scan.
Conclusion:
68Ga-DOTATATE PET/CT scan is more useful than CECT scan for the early detection of bone metastases in pediatric NETs.
Keywords: 68Ga-DOTATATE positron emission tomography, bone metastases, computed tomography, pediatric neuroendocrine tumors
INTRODUCTION
Endocrine and neuroendocrine cells form a large and diverse array of cell types which are present in the form of specialized organs, such as the pituitary, parathyroid, thyroid and adrenal gland and in the form of the diffuse neuroendocrine system in the respiratory and digestive tracts.[1] Neuroendocrine tumors (NETs) arising from the neural crest, such as neuroblastomas, form a large proportion of childhood malignancies, accounting for 7% to 10% of all pediatric neoplasms.[2] Gastroenteropancreatic-NETs (GEP-NETs) form a small subgroup of neural crest tumors whose imaging findings are not well-described in children. These tumors arise from neuroendocrine cells in the fore gut (with its derivatives including the bronchial tree), midgut, hind gut and pancreas. Although NETs form a heterogeneous group of neoplasms, yet these present with certain unifying features including frequent hormonal overproduction that leads to specific symptoms and a typical immunohistochemical staining profile with chromogranin-A and synaptophysin reactivity.[1] Certain tumors occur as part of hereditary syndromes such as multiple endocrine neoplasia types 1 and 2, Von Hippel-Lindau disease, neurofibromatosis type 1, Carney complex, pheochromocytoma-paraganglioma syndrome and familial medullary thyroid carcinoma. These syndromes generally appear at a young age and are characterized by specific genetic abnormalities that have proved helpful in our understanding of the process of tumorigenesis.
Although these tumors can produce distinct clinical syndromes due to their secretory capacity, they are under diagnosed in children, resulting in delays in detection.[3] Most of the information regarding imaging of these tumors is obtained from adult studies using various anatomic imaging modalities such as multi-detector computed tomography (CT), magnetic resonance imaging (MRI), sonography and endoscopic ultrasonography with color Doppler. Most NETs overexpress somatostatin receptors and also possess amine uptake and storage mechanisms, allowing targeted molecular imaging using somatostatin receptor scintigraphy (SRS) and metaiodobenzylguanidine (MIBG), respectively. Traditionally 111In-diethylenetriaminepentaacetic acid (DTPA) octreotide scintigraphy has been used. SRS using 68Ga-DOTATATE is a highly sensitive and specific imaging modality for detection and staging of NETs.[4] Other positron emission tomography (PET) tracers such as 18Fluorodeoxyglucose, which though widely used tracer, but has limited sensitivity in detection of well differentiated and slow-growing tumors and 6-[fluoride-18] fluoro-levodopa (18F-dihydroxyphenylalanine (DOPA) have also been investigated.[5,6]
Bone scintigraphy is very sensitive for the detection of bone metastases. The findings of conventional bone scintigraphy depend on perfusion within the bone and also on its osteoblastic activity. Furthermore, bone-seeking agents are unable to detect bone marrow involvement if there is no significant bone reaction.[7] MRI of regions of interest is the most sensitive modality for detecting bone metastases in NET however whole body MRI is a difficult proposition.[8]
Bone metastases in patients with advanced NETs are associated with a poor prognosis and form a contraindication for extended surgical resection.[9] Early detection of bone metastases is thus warranted. Since imaging findings of NETs are not well described in children and because of limited information available in the pediatric literature we aimed at evaluating the usefulness of 68Ga-DOTATATE PET/CT scan for detection of bone metastases in pediatric NETs and compared its findings with the CT scan.
MATERIALS AND METHODS
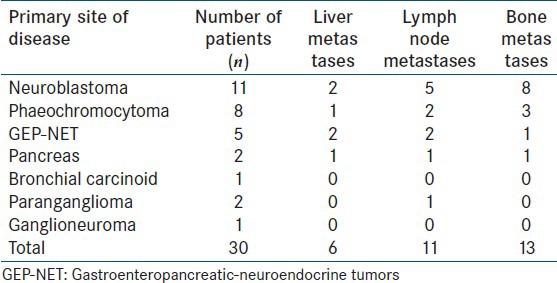
A retrospective analysis of 30 patients (18 males 12 females; age range: 1-18 years; mean age 7.8 years) with histologically confirmed NETs who underwent 68Ga-DOTATATE PET/CT scan for primary staging was done. The study included 11 neuroblastomas, 8 phaeochromocytomas, 5 GEP-NETs, 2 pancreatic NETs, 2 paragangliomas, 1 bronchial carcinoid and 1 ganglioneuroma [Table 1]. All patients underwent 68Ga-DOTATATE PET/CT scan at the time of diagnosis for primary staging. Contrast enhanced CT (CECT) performed at the time of PET scan acquisition was used for comparison with PET data.
Table 1.
Patient details
The tracer was prepared in house using ITG (Isotope Technologies Garching GmbH) 68Ge/68Ga generator in manual module. 18-74 MBq of radioactivity of 68Ga-DOTATATE was injected intravenously in each patient. All the imaging studies were acquired by using a dual modality PET/CT (Discovery STE-16, GE health-care, Milwaukee, USA). Approximately 45-60 min after injection, static 68Ga-DOTATATE PET/CT imaging in 3-D mode from head to toe was acquired in all patients. 3-D PET acquisition was done for 2 min per bed position for three-five bed positions. PET data was acquired using matrix of 128 × 128 pixels with a slice thickness of 1.5 mm. For iterative reconstruction, 2 iterations and 26 subsets were used, with an inter update filter of 4 mm in full width at half maximum and a post processing filter of 6 mm in full width at half maximum. CT based attenuation correction of the emission images was employed. PET images were reconstructed by iterative method ordered subset expectation maximization (OSEM). In the PET/CT system, CT scan acquisition was performed on 16 slice CT using intravenous contrast with 120 kV, 40 mA, rotation time of 0.5 s and slice thickness of 3.25 mm in the age group of 0-3 years, 120 kV, 60 mA, rotation time of 0.5 s and slice thickness of 3.25 mm in age group of 3-6 years and 120 kV, 70 mA, rotation time of 0.5 s and slice thickness of 3.25 mm in age group of 6-12 years for diagnostic CT acquired with 68Ga-DOTATATE PET/CT scan.
Imaging results were analyzed on a per-patient and on a per-lesion basis. The effectiveness of 68Ga-DOTATATE PET and CT in defining bone involvement was assessed in the following locations: Skull, orbit, axial skeleton and appendicular skeleton. Follow-up of the patients was done clinically as well as with subsequent PET/CT imaging wherever possible.
Statistical analysis
Descriptive statistics was used for the variables as needed. Difference between the number of lesions detected by both imaging modalities was assessed by Wilcoxon signed rank test whereas McNemar's test was done to evaluate differences on a per patient basis. For lesion wise disease detection histopathological diagnosis was not available, so, inter rater kappa agreement was used to evaluate degree of agreement between both types of functional scans. P <0.05 was considered to be statistically significant.
RESULTS
Out of 30 patients, 17 had no evidence of bone metastases on any imaging modality or on clinical follow-up whereas 13 patients showed evidence of bone metastases. Of these 13 patients, there were eight cases of neuroblastoma, three of phaeochromocytoma, one of GEP-NET and one of pancreatic NET [Table 2]. All 13 patients showed positivity on 68Ga-DOTATATE PET for metastatic bone lesions whereas CT scan was positive in nine patients. Of the four patients who were negative on CT scan, three were cases of neuroblastoma (two with axial and appendicular skeletal metastases and one with only appendicular skeletal metastasis) and one was a case of phaeochromocytoma (with sternal, femoral and vertebral metastases). However, the inter rate agreement (kappa) was high with kappa co efficient value of 0.773 (P < 0.001).
Table 2.
Summary of the primary findings of patients with bone metastases
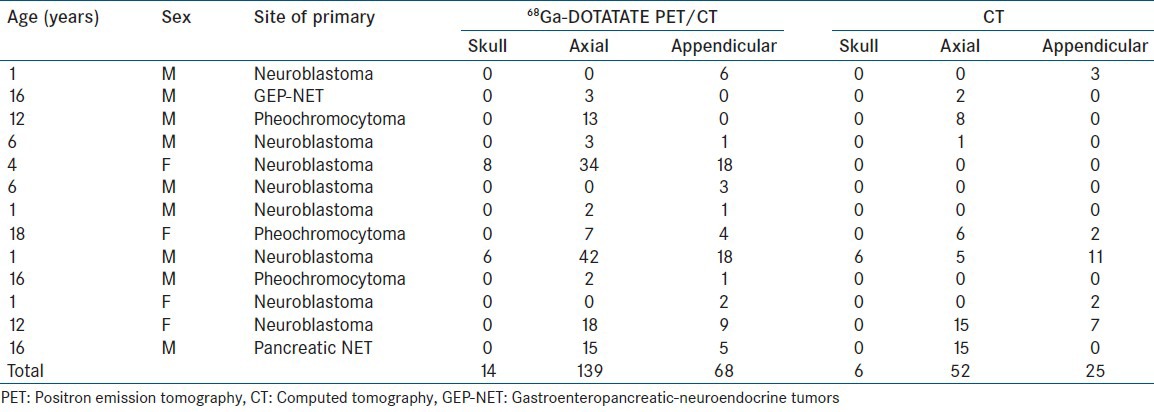
Four patients showed diffuse osseous spread throughout the body of which two were cases of neuroblastoma, one was a case of pheochromocytoma and one was a case of pancreatic NET. Skull metastases was seen in two cases of neuroblastoma, metastases to the spine in 8 cases (3 neuroblastoma, 1 GEP-NET, 3 phaeochromocytoma and 1 pancreatic NET) and appendicular bone metastases were seen in 11 patients (8 neuroblastoma, 2 phaecochromocytoma, 1 pancreatic NET).
On a lesion wise analysis, of the 225 total bone lesions detected on 68Ga-DOTATATE PET, 14 were detected in the skull, four in the orbit, 139 in the axial skeleton and 68 in the appendicular skeleton. In comparison, CT scan alone detected only 84 bone lesions. No additional/extra lesions were detected by CT scan. Of these 84 lesions, there were six in skull, one in orbit, 52 in axial skeleton and 25 in appendicular skeleton. Difference between the total number of lesions, axial and appendicular skeletal lesions was found to be significant by Wilcoxon signed rank test (P = 0.012; P = 0.014; P = 0.002, respectively).
68Ga-DOTATATE PET detected the primary site in 27 patients. However no tracer uptake was noted at the primary site in three patients (one case of phaeochromocytoma and two cases of GEP-NET). Of the 13 patients with bone metastases, five patients had lymph node metastases (3 neuroblastoma; 1 GEP-NET; 1 pancreatic NET) and four patients had liver metastases (2 neuroblastoma; 1 GEP-NET; 1 pancreatic NET).
DISCUSSION
NETs are rare diseases, but their incidence is believed to be higher than reported, primarily because of a number of tumors that go undetected, especially when they are small and clinically silent.[10] Most NET expresses a high density of somatostatin receptors, so they can be successfully localized in-vivo by SRS. Since PET has superior imaging characteristics, its use improves the detection of NETs which has also been proven by many clinical studies especially those with the use of 68Ga-DOTATATE PET[11] but literature on the use of 68Ga-DOTATATE PET in pediatric NETs per se is lacking. Bone metastases in NET confers poor prognosis, warranting its early detection in the management of therapy, allowing it to begin earlier or to be changed to symptomatic palliation.[12]
In our study, the sensitivity of the conventional CT scan for the detection of bone metastases was found to be low as has also been demonstrated in a study by Putzer et al.[13] CT scan failed to pick up bone metastases in three patients of neuroblastoma and in one patient of phaeochromocytoma largely because radiographic signs of bone involvement can be very subtle and easily get missed. 68Ga-DOTATATE was useful in the characterization and upstaged the disease to stage IV, changing the management plan in a 4 year female child who was referred as a suspected case of neuroblastoma and did not show any bone involvement on the CT scan [Figure 1]. Similarly, in another patient of neuroblastoma, the presence of bone metastases upstaged the disease from stage I to stage IV [Figure 2]. 68Ga-DOTATATE PET scanning in a 16-year-old boy diagnosed with phaeochromocytoma revealed multiple sites of bone metastases which were otherwise missed by CT scan [Figure 3]. Axial skeleton is the most common site of bone metastases in phaeochromocytoma[14] similar to the findings in our study.
Figure 1.
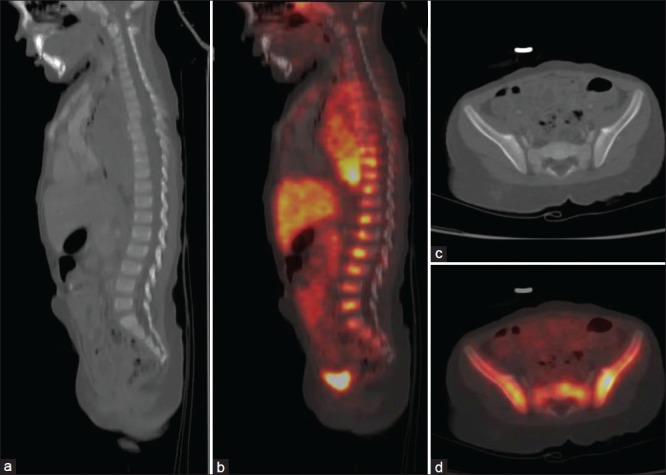
A 4-year-old female child with suspected neuroblastoma showed multiple intensely 68Ga-DOTATATE avid foci in dorso-lumbar vertebrae (b) Pelvis (d) With no definite changes on computed tomography (a and c) Upstaging the disease to stage IV
Figure 2.
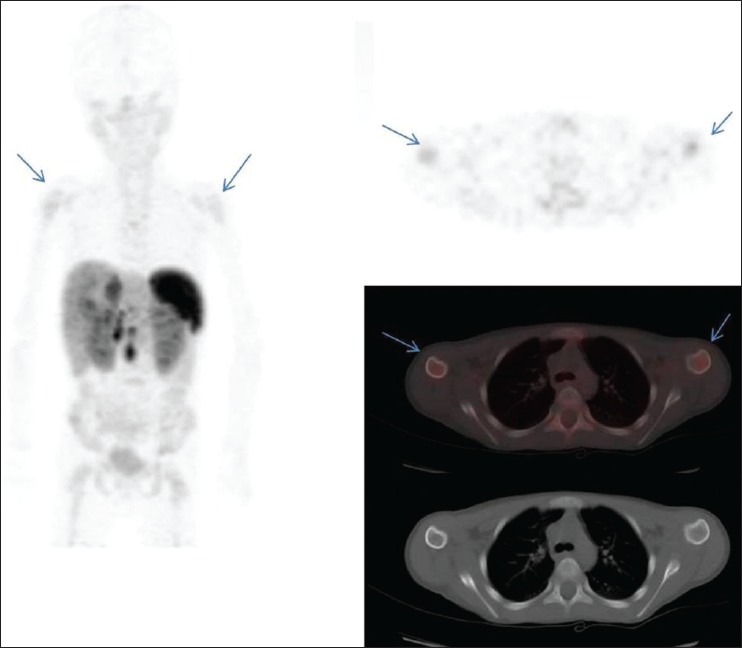
A 6-year-old male child with diagnosed neuroblastoma showed moderate 68Ga-DOTATATE avidity in bilateral humeral heads (arrows) with no definite changes on computed tomography
Figure 3.
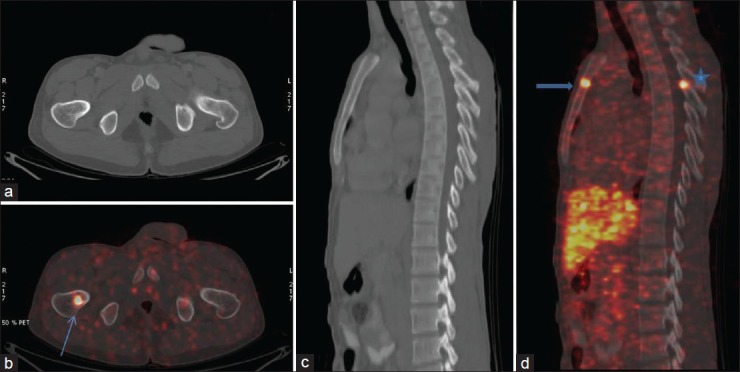
A 16-year-old boy diagnosed with pheochromocytoma underwent 68Ga-DOTATATE positron emission tomography/computed tomography (CT). Intense 68Ga-DOTATATE avid focus is noted in right femur (arrow) with no definite changes on CT. In the right hand panel foci of Ga68 avidity are seen in sternum (arrow head) and D6 vertebral posterior elements*, again with no definite changes on CT images (c) On follow-up confirmed to be skeletal metastases
Other than neuroblastoma and phaeochromocytoma, pancreatic NET and GEP-NETs (small bowel) also showed evidence of bone metastases in our study. Imaging findings of bone metastases in these malignancies has been previously described with bone metastases mostly occurring in patients with liver metastases.[15] In our study also both patients of GEP-NET and pancreatic NET with bone metastases had liver metastases. Several studies have reported bone metastases in 7-15% of patients with NETs.[8,16,17] However, the data had been generated from adult studies. Our study showed a high incidence with 43% (13/30) patients having evidence of bone metastases most likely because the patients were already in late stages of disease at the time of referral. MRI is found to be the most sensitive modality for the detection of bone metastases in patients with NET.[8] However, only regional MRI scans of parts of the body suspected of having bone metastases are generally performed. None of the patients in our study underwent MRI for detection of bone metastases.
Pain is the main symptom in patients with NETs who have slowly growing metastases to the axial skeleton[18] however, younger patients do not complain of pain and it's difficult to be accurately elicited, thus emphasizing the importance of highly sensitive imaging modalities as reliable indicators of distant metastases in pediatric patients.
Although histopathological diagnosis could not be obtained from the sites of bone metastases, all patients were followed-up clinically and additionally in some patients, PET findings were confirmed by PET/CT follow-up 6 months after initial staging (n = 10) showing either progression or disappearance of lesions with clinical co-relation. Due to lack of histopathological correlation, lesion wise sensitivity and specificity was not calculated.
We did not compare the findings of our study with 99mTechnetium-methylene diphosphonate bone scintigraphy; however, it has already been proven that 68Ga-DOTA labeled peptide PET is far more sensitive than conventional nuclear medicine imaging for bone metastases by several studies.[4,19,20] In our study, 68Ga-DOTATATE PET/CT scan showed significantly more bone lesions in comparison to CT scan thus making it the best available tracer for detection of bone metastases in NETs of childhood.
CONCLUSION
Our study indicates that 68Ga-DOTATATE PET/CT is more useful than CECT alone for the early detection of bone metastases in pediatric NETs with clear benefits in patients of neuroblastoma and pheochromocytoma. However larger multicenter prospective studies are needed to further validate its role in these childhood malignancies.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gaal J, de Krijger RR. Neuroendocrine tumors and tumor syndromes in childhood. Pediatr Dev Pathol. 2010;13:427–41. doi: 10.2350/09-04-0635-CP.1. [DOI] [PubMed] [Google Scholar]
- 2.Castleberry RP. Neuroblastoma. Eur J Cancer. 1997;33:1430–7. doi: 10.1016/s0959-8049(97)00308-0. [DOI] [PubMed] [Google Scholar]
- 3.Khanna G, O’Dorisio SM, Menda Y, Kirby P, Kao S, Sato Y. Gastroenteropancreatic neuroendocrine tumors in children and young adults. Pediatr Radiol. 2008;38:251–9. doi: 10.1007/s00247-007-0564-4. 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–18. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 5.Fiebrich HB, Brouwers AH, Kerstens MN, Pijl ME, Kema IP, de Jong JR, et al. 6-[F-18]Fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to conventional imaging with (123) I-metaiodobenzylguanidine scintigraphy, computer tomography, and magnetic resonance imaging in localizing tumors causing catecholamine excess. J Clin Endocrinol Metab. 2009;94:3922–30. doi: 10.1210/jc.2009-1054. [DOI] [PubMed] [Google Scholar]
- 6.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–67. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman JM, Plonk JW. 99mTc-pyrophosphate bone scans in patients with metastatic carcinoid tumors. J Med. 1977;8:71–80. [PubMed] [Google Scholar]
- 8.Gibril F, Doppman JL, Reynolds JC, Chen CC, Sutliff VE, Yu F, et al. Bone metastases in patients with gastrinomas: A prospective study of bone scanning, somatostatin receptor scanning, and magnetic resonance image in their detection, frequency, location, and effect of their detection on management. J Clin Oncol. 1998;16:1040–53. doi: 10.1200/JCO.1998.16.3.1040. [DOI] [PubMed] [Google Scholar]
- 9.Panzuto F, Nasoni S, Falconi M, Corleto VD, Capurso G, Cassetta S, et al. Prognostic factors and survival in endocrine tumor patients: Comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005;12:1083–92. doi: 10.1677/erc.1.01017. [DOI] [PubMed] [Google Scholar]
- 10.Illouz F, Sadoul JL, Rohmer V. Somatostatin receptor-based imaging and therapy of digestive endocrine tumors. Ann Endocrinol (Paris) 2010;71(Suppl 1):S3–12. doi: 10.1016/S0003-4266(10)70002-0. [DOI] [PubMed] [Google Scholar]
- 11.Rufini V, Calcagni ML, Baum RP. Imaging of neuroendocrine tumors. Semin Nucl Med. 2006;36:228–47. doi: 10.1053/j.semnuclmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Lebtahi R, Cadiot G, Delahaye N, Genin R, Daou D, Peker MC, et al. Detection of bone metastases in patients with endocrine gastroenteropancreatic tumors: Bone scintigraphy compared with somatostatin receptor scintigraphy. J Nucl Med. 1999;40:1602–8. [PubMed] [Google Scholar]
- 13.Putzer D, Gabriel M, Henninger B, Kendler D, Uprimny C, Dobrozemsky G, et al. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J Nucl Med. 2009;50:1214–21. doi: 10.2967/jnumed.108.060236. [DOI] [PubMed] [Google Scholar]
- 14.Lynn MD, Braunstein EM, Wahl RL, Shapiro B, Gross MD, Rabbani R. Bone metastases in pheochromocytoma: Comparative studies of efficacy of imaging. Radiology. 1986;160:701–6. doi: 10.1148/radiology.160.3.3737909. [DOI] [PubMed] [Google Scholar]
- 15.Debray MP, Geoffroy O, Laissy JP, Lebtahi R, Silbermann-Hoffman O, Henry-Feugeas MC, et al. Imaging appearances of metastases from neuroendocrine tumours of the pancreas. Br J Radiol. 2001;74:1065–70. doi: 10.1259/bjr.74.887.741065. [DOI] [PubMed] [Google Scholar]
- 16.Kwekkeboom DJ, Krenning EP, Bakker WH, Oei HY, Kooij PP, Lamberts SW. Somatostatin analogue scintigraphy in carcinoid tumours. Eur J Nucl Med. 1993;20:283–92. doi: 10.1007/BF00169802. [DOI] [PubMed] [Google Scholar]
- 17.Westlin JE, Janson ET, Arnberg H, Ahlström H, Oberg K, Nilsson S. Somatostatin receptor scintigraphy of carcinoid tumours using the [111In-DTPA-D-Phe1]-octreotide. Acta Oncol. 1993;32:783–6. doi: 10.3109/02841869309096136. [DOI] [PubMed] [Google Scholar]
- 18.Meijer WG, van der Veer E, Jager PL, van der Jagt EJ, Piers BA, Kema IP, et al. Bone metastases in carcinoid tumors: Clinical features, imaging characteristics, and markers of bone metabolism. J Nucl Med. 2003;44:184–91. [PubMed] [Google Scholar]
- 19.Buchmann I, Henze M, Engelbrecht S, Eisenhut M, Runz A, Schäfer M, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34:1617–26. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 20.Kroiss A, Putzer D, Uprimny C, Decristoforo C, Gabriel M, Santner W, et al. Functional imaging in phaeochromocytoma and neuroblastoma with 68Ga-DOTA-Tyr 3-octreotide positron emission tomography and 123I-metaiodobenzylguanidine. Eur J Nucl Med Mol Imaging. 2011;38:865–73. doi: 10.1007/s00259-010-1720-x. [DOI] [PubMed] [Google Scholar]


