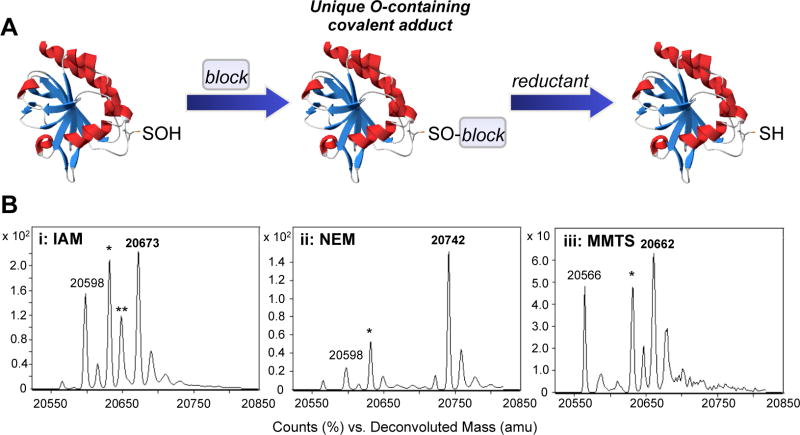
Figure 2.
A. General reaction scheme for AhpC-SOH addition to thiol-blocking electrophiles. B. Representative ESI-TOF mass spectra of the reactions of C165S AhpC-SOH (30 μM) with 2 mM IAM (i), NEM (ii), and MMTS (iii) at 3 hours at pH 7.5. The signal at 20,598 amu corresponds to sulfenamide (-SN). Signals labeled with * and ** indicate 20,632 (-SO2H) and 20,648 (-SO3H) a.m.u., respectively. Evidence for nucleophilic -SOH addition is indicated at 20,673 (i), 20,742 (ii), and 20,662 (iii) a.m.u. The signal at 20,566 a.m.u. (iii) corresponds to the conversion of cysteine to dehydroalanine.