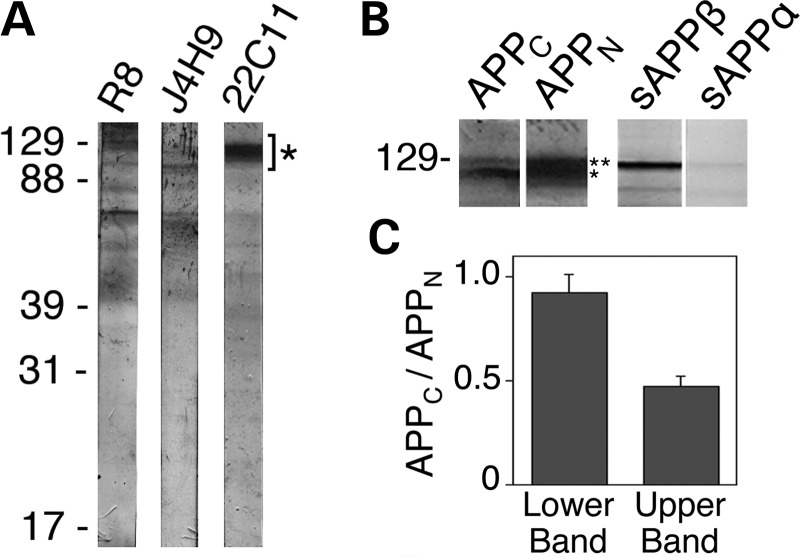
Figure 2.
APP-derived NTFs reside within neurons. (A) Immunoblot analysis of CAD cell extracts with antibodies raised against polypeptides from the ectodomain of APP (see Fig. 1A for location of the recognized epitopes). Full-length APP species migrate to positions indicated by (*). All antibodies also detect polypeptides of lower molecular size; as many of these additional bands are detected by all antibodies, they likely are bona fide cleavage products of APP or sAPP. The position of molecular size markers (in kDa) is shown at left. (B) Immunoblots of CAD cell extracts with antibodies to polypeptides from the C-terminal (APPC) and N-terminal (APPN; 22C11) regions of APP, and with antibodies detecting the free C-termini of sAPPβ or sAPPα, which do not cross-react with full-length APP. Note that CAD cell extracts contain genuine sAPPβ, which co-migrates with full-length APP species. Notably, under our SDS–PAGE conditions, sAPPβ has a lower electrophoretic mobility compared with the immature APP species, but higher than the mature, full-length APP. (C) Histogram, derived from western blots, showing the ratios of the staining intensities with the antibodies to APPC and APPN (APPC/APPN) for the lower (*) and upper (**) bands in the APP region (see B). Note that the ratios differ significantly and indicate that the upper band includes APP species that contain the N-terminal epitope, but lack the C-terminal epitope. The histogram indicates that approximately half of the staining with antibody 22C11 in the upper band may represent high molecular size NTFs, such as sAPPβ. Bars represent SD.