FIGURE 3.
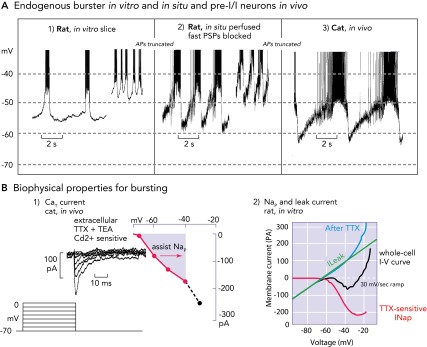
Biophysical properties and bursting behavior of pre-BötC inspiratory neurons
A: subpopulations of rodent pre-BötC neurons exhibit voltage-dependent endogenous rhythmic bursting in vitro (A1) after blockade of fast excitatory synaptic transmission (adapted from Ref. 36 and used with permission) or in situ (A2) after blockade of fast synaptic transmission (93). The membrane potentials for onset of rhythmic bursting in all cases is −60 mV, and the bursting frequency increases progressively with depolarization up to a baseline at approximately −45 mV. Identified eBNs reveal similar interburst voltage trajectories as recorded from pre-I/I neurons in vivo (A3), some of which may have intrinsic bursting behavior (58, 64). Recorded in vivo, these neurons also start to depolarize for bursting at a voltage range of approximately −60 mV. As synaptic interactions are intact in vivo, the membrane potential trajectory is controlled by postsynaptic inhibition during the post-inspiratory phase, during which endogenous bursting is suppressed. All action potentials are truncated. B1: when conditioned with hyperpolarizing pre-pulses, all groups of respiratory neurons generate a CaT current already at negative voltages of −80 mV that can depolarize neurons to spike activation threshold (64). B2: the underlying biophysical properties for in vitro and in situ endogenous bursting are primarily a TTX-sensitive persistent Na+ channel (Nap) and an ohmic-like Kleak conductance (34). The Nap current-voltage (I-V) relation (red curve), obtained from a slow voltage clamp ramp protocol applied to in vitro eBNs, is revealed after blocking the current with TTX and subtracting the resultant I-V relation from the whole cell I-V curve measured before TTX application. The K+-dominated leak I-V relation (green line) is obtained from the linear region of the whole-cell I-V curve (after Ref. 34).
