Abstract
Background:
Recent studies have demonstrated that during chronic Helicobacter pylori (H. pylori) infection bone marrow-derived-mesenchymal stem cells (BMD-MSCs) migrate to the gastric tissue and could be also the origin of gastric adenocarcinoma. The chemokine CXCR4 through binding to its ligand stromal-derived factor (SDF-1) plays a crucial role in migration of inflammatory and stem cells. However, the possible effect of H. pylori infection on the SDF-1/CXCR4 axis has not yet been elucidated.
Materials and Methods:
Gastric epithelial cell line, AGS, and BMD-MSCs were cocultured with H. pylori for 24 h. The expression of CXCR4 was examined in BMD-MSCs by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and flow cytometry, and SDF-1 expression in AGS cells was detected by qRT-PCR and enzyme-linked immunosorbent assay. Further, migration of BMD-MSCs toward SDF-1 was evaluated by chemotaxis assay.
Results:
We found that coculture of H. pylori with BMD-MSCs or AGS: (i) enhanced CXCR4 expression on the cell surface of BMD-MSCs and (ii) increased SDF-1 secretion by AGS cells. Consistently, we observed that H. pylori-treated BMD-MSCs showed a higher capability to migrate toward SDF-1 gradient compared with untreated cells.
Conclusion:
We found that H. pylori upregulates CXCR4 expression in BMD-MSCs and enhance their migration toward SDF-1. This study provides the first evidence that H. pylori infection may enhance BMD-MSC migration through acting on the SDF-1/CXCR4 axis.
Keywords: CXCR4, Helicobacter pylori, gastric cancer, mesenchymal stem cells
INTRODUCTION
Helicobacter pylori (H. pylori) selectively colonizes gastric epithelium and is the most common bacterial infection worldwide.[1] Increasing evidence indicates that H. pylori is the strongest risk factor for malignancies that arise within the stomach.[2] Because of clinical relevance, the World Health Organization (WHO) has classified H. pylori as a class I carcinogen for gastric cancer.[3,4] H. pylori adheres to gastric cells and by virulence factors such as vacA and cagA causes damage to cells.[5,6] Although the exact carcinogenesis mechanism of H. pylori is unknown, accumulating evidence indicate that high amount of reactive oxygen and nitric oxide, which react with nuclear DNA and cause different mutations in the genes, ultimately lead to the accumulation of DNA damage, genetic defects, and appearance of malignant cells.[4,7]
Recent studies showed that bone marrow-derived mesenchymal stem cells (BMD-MSCs) are multipotent cells and are able to migrate cross tissue to differentiate into many cell types including tumor cells.[8,9,10] In general, whenever tissue injury occurs stem cell particularly BMD-MSCs start to repair the damaged tissue. However, in the case of chronic inflammation due to persistence of H. pylori infection in stomach, the local stem cells fail to repair the injured tissue. This may allow BMD-MSCs to migrate and engraft within gastric stem cell niches. Once BMD-MSCs engrafted, and because of H. pylori persistence, these cells are exposed to many H. pylori-derived substances and various inflammatory factors which is likely to drive their transformation to gastric adenocarcinoma.[10,11] Nevertheless, the specific molecular mechanism of migration and homing of BMD-MSCs into the gastric tissue have not been yet elucidated.
Chemokines are a superfamily of small peptides that are involved in many physiological processes such as cell trafficking. These molecules mediate the migration of leukocytes in inflammatory sites and most of them are increased during infection and inflammation.[12,13] The stromal-derived factor-1 (SDF-1) and its receptor, CXCR4, play an important role in retention and engraftment of hematopoietic stem cells (HSCs) within the bone marrow[14] as well as metastasis of various tumors such as gastric, breast, and lung cancers.[15,16] The expression of CXCR4 on many cancer cells implies that the SDF-1/CXCR4 axis influences cancer biology and plays a crucial role in metastasis of CXCR4-expressing cells to target tissues that produce a high level of SDF-1.[17] Furthermore, SDF-1 and CXCR4 have been shown to be upregulated during hypoxia and tissue injury.[18,19,20] Liu et al. have recently shown that preconditioning of MSCs in hypoxia resulted in CXCR4 upregulation and increased recruitment of these cells to ischemic kidney, which express a high amount of SDF-1 than normal kidney[18]. Moreover, a recent study reported that the levels of CXCR4 in H. pylori-positive gastric cancer were significantly higher than those with H. pylori.[21] In the current study, we aimed to examine the possible effect of H. pylori infection on the SDF-1/CXCR4 axis in BMD-MSCs. We have found that H. pylori significantly enhances CXCR4 expression in BMD-MSCs and these H. pylori treated cells show a better response to SDF-1 gradient.
MATERIALS AND METHODS
Bacterial culture
H. pylori bacteria were isolated from clinical biopsy of a patient with peptic ulcer. Single colonies of H. pylori strain were isolated and confirmed for identity according to colony morphology, wet mount, and microscopic observation after Gram staining and biochemical analysis (urease and catalase tests). H. pylori strains were cultured on brucella agar plates supplemented with sheep blood (10% v/v), fetal bovine serum (FBS) (7% v/v), and antibiotics (10 mg/L of vancomycin, 2 mg/L of amphothericin B, 50 mg/L of polymyxin B) in a microaerophilic gas mixture composed of 5% O2, 10% CO2, and 85% N2 at high humidity. DNA was extracted from bacteria and the presence of cagA, vacA, and urease genes were analyzed by PCR using the primers. Primer sequences were designed using Gene Runner software (http://www.generunner.net) and are listed in Table 1.
Table 1.
Primer sequences for PCR

Cell culture
The AGS cell line (human gastric adenocarcinoma cell line) was purchased from Iran Pasteur Institute (Tehran, Iran) and were cultured in RPMI 1640 supplemented with 10% FBS (Gibco, Manchester, UK) at 37°C in a humid incubator with 5% CO2. Human BMD-MSCs were expanded in vitro from the bone marrow of healthy donors after informed consent was obtained. Mononuclear cells (MNCs) were isolated by gradient centrifugation at 2,500 rpm for 30 min on Ficoll-Paque™ Plus (Amersham Pharmacia Biotech, Uppsala, Sweden). Then, plated at a concentration of 20-30 × 106 cells/cm2 in Dulbecco's Modified Eagle Medium (DMEM) containing 20% (v/v) of FBS. Then, nonadherent cells were removed 2 days later and a fresh medium was added. BMD-MSCs were trypsinized when the cultures reached 80-100% confluence and subcultured. The purity of MSC suspensions was assessed by flow cytometry using the following monoclonal antibodies: anti-CD34-FITC, CD45-FITC, CD73-FITC (all from Biolegend, Ankara, Turkey), and Stro-1 (R&D, Istanbul, Turkey).
Bacterial coculture
First, the H. pylori colonies were collected and suspended in culture medium and the optical density (OD) was adjusted to one absorbance units at 600 nm, corresponding to a bacterial concentration of 2 × 108 CFU/mL. Then, H. pylori bacteria were added to the subconfluent BMD-MSCs or AGS cells in culture media without FBS in 25-cm2 tissue culture flasks (the bacteria-to-cell ratio was approximately 100:1) and cultured in a 5% CO2 incubator at 37°C for 24 h. Cells were then harvested and used for flow cytometry and quantitative reverse transcription polymerase chain reaction (qRT-PCR) assays.
Reverse transcription polymerase chain reaction and quantitative reverse transcription polymerase chain reaction
Expression of mRNA for CXCR4 and SDF-1 was evaluated in BMD-MSCs and gastric epithelial cells, respectively. RNA was isolated using a RNA extraction kit (Bioflux, Basel, Switzerland) and RNA was transcripted into cDNA using of Bioneerkit (Bioneer, Daejeon, South Korea). Reverse transcription-polymerase chain reaction (RT-PCR) procedures were carried out using primer (designed by Gene Runner software) sequences as listed in Table 2. Amplification was performed with a thermocycler (Mastercycler, Eppendorf, Westbury, NY) and the PCR products were electrophoresed on a 2% agarose gel containing ethidium bromide. The PCR products were visualized by a gel document system.
Table 2.
Primer sequences for qRT-PCR

Real-time quantitative reverse transcription PCR (real-time qRT-PCR) was performed in a sequence detection system (Rotor-Gene™ 6000, Corbett Life Science, Sydney, Australia) and carried out using SYBR Green dye detection protocol. Relative quantitation of CXCR4 and SDF-1 mRNA expression was calculated using the comparative CT method.[22] The relative quantitation value of mRNA expression for CXCR4 and SDF-1 was normalized to an endogenous control β-actin gene as previously reported.
ELISA assay
The concentration of SDF-1 levels were examined using an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer instruction (Ray Bio, Middlesex, UK). Supernatants were collected 24 h after coculture of AGS cells and H. pylori, centrifuged at 11,000 rpm for 10 min to discard bacteria and cell debris, and stored at −80°C until assayed.
Fluorescence-activated cell sorting analysis
BMD-MSCs were cocultured with H. pylori for 24 h, harvested, and washed with FCM buffer (PBS containing 1% BSA) three times. Cells were stained as described previously.[23] Briefly, cells were incubated with 10 μg/mL of mouse anti-human CXCR4 (Santa Cruz, Dallas, TX) or isotype antibody (Dako, Glostrup, Denmark) for 45 min on ice, then washed and incubated with goat anti-mouse FITC conjugated secondary antibody (Dako) for 30 min. Cells then were washed three times, fixed in 1% paraformaldehyde, and subjected to FACS analysis (FACS Calibur, Beckman Dickinson, San Jose, CA). The purity of BMD-MSCs was determined using stained anti-CD45-FITC, CD73-FITC, CD34-FITC, and Stro-1. Cells were incubated with 10 μL of either anti-CD45-FITC, CD73-FITC, or CD34 FITC for 30 min at room temperature, washed three times with FCM buffer, and subjected to FACS analysis. To stain BMD-MSCs for Stro-1 (R&D, Minneapolis, MN) cells were incubated with anti-Stro-1 monoclonal antibody for 45 min on ice, then washed and incubated with secondary antibody as mentioned above. FACS data were analyzed by FCS Express software (De Novo Software, Los Angeles, CA).
Chemotaxis assay
The chemotaxis assay was performed as described previously.[24] Cell culture inserts with 8-μm pore filters (Greiner Bio-One, Monroe, NC) were used to measure chemotaxis. H. pylori-treated or untreated MSCs (50 × 103 cells) were added to the upper chamber, and 600 μL of serum-free DMEM with or without 100 ng/mL SDF-1 (PeproTech, Hamburg, Germany) was added to the bottom chamber. Cells were incubated for 6 h at 37°C and then cells remaining on the upper face of the chambers were removed with a cotton swap. Migrated cells were evaluated on the undersides of filters after being fixed with methanol and stained with 2% toluidine. Five random fields were selected for microscopic count using a 20× objective and light microscopy.
Statistical analysis
Arithmetic means and standard deviations were calculated and statistical significance was defined as P ≤ 0.05 using Student's t-test.
RESULTS
Characterization of isolated H. pylori
To determine the virulence factors of H. pylori, bacteria were cultured on brucella agar for 48 h, then harvested and virulence factors were examined by PCR. All the biochemical and microbiological tests confirmed that the isolated bacteria are H. pylori (data not shown). The isolated bacteria contain the genes for vacA and urease; however, cagA is absent [Figure 1].
Figure 1.
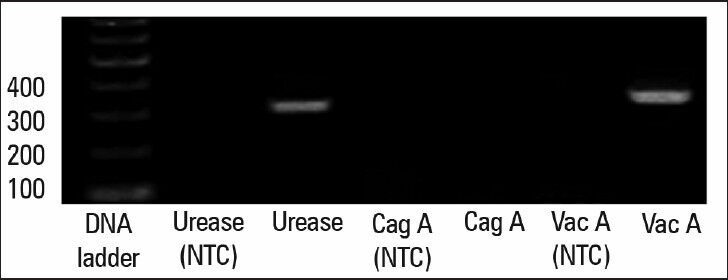
Determination of H. pylori virulence factors. PCR results confirmed the existence of the two genes (vacA and urease) and the absence of the cagA gene. Nuclease-free water used for nontemplate control (NTC)
Characterization of BMD-MSCs
Morphological studies shown that our BMD-MSCs are elongated fibroblast-like shape, which is one the characteristic of BMD-MSC [Figure 2a]. To examine the purity of isolated BMD-MSCs, the cells were stained with specific markers for MSCs and hematopoietic cells. As shown in Figure 2b, our BMD-MSCs do not express CD34 and CD45, which are the markers of hematopoietic cells. In contrast, these cells are positive for STRO-1 and CD73, indicating that our BMD-MSCs are pure MSCs and are not mixed with any hematopoietic cells.
Figure 2.
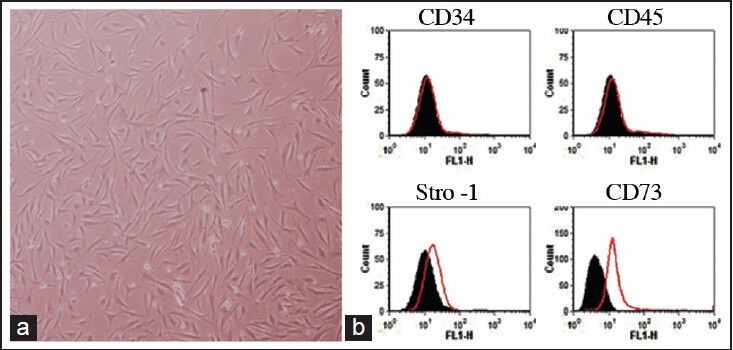
Characterization of BMD-MSCs. (a) Morphological evaluation of BMD-MSCs in passage five. (b) Representative FACS analysis shows that BMD-MSCs are negative for hematopoietic markers (CD34 and CD45), but are positive for MSC markers (Stro-1 and CD73). Black-filled histogram shows isotype control antibody and red histograms show CD34, CD45, Stro-1, or CD73 antigens
H. pylori infection enhances SDF-1 expression in gastric epithelial cells
SDF-1 is a ubiquitous chemokine which is expressed in many cells including gastric epithelial cells, but the possible effect of H. pylori infection on SDF-1 expression in these cells has not been yet explored.[25] In this study, we infected gastric epithelial cell line, AGS, with H. pylori for 24 h and by using semi quantitative gel-based RT-PCR and qRT-PCR, we found that SDF-1 is expressed in AGS cells and is significantly upregulated (2.3-fold; P < 0.02) by infection with H. pylori [Figure 3a–b]. Consistently, we observed that SDF-1 is produced by untreated AGS cells (111 pg/mL ± 33) and that SDF-1 production is significantly increased (3.15-fold; P < 0.018) when the cells were infected with H. pylori (338 pg/mL ± 72) [Figure 3c], confirming the RT-PCR results.
Figure 3.
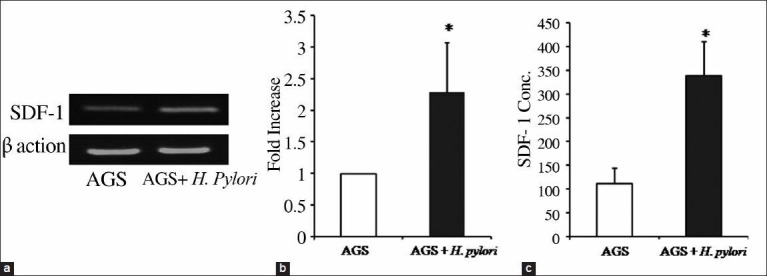
The effect of H. pylori infection on SDF-1 expression in AGS. AGS cells were infected with H. pylori for 24 h, then mRNA was isolated, and SDF-1 expression was examined by qRT-PCR. (a) RT-PCR analysis of mRNA transcripts for SDF-1 in noninfected and H. pylori-infected AGS cells. β-actin was used as internal loading control for the RT-PCR. (b) qRT-PCR shows that SDF-1 is significantly increased in H. pylori-infected cells. SDF-1 expression was normalized to an endogenous control β-actin gene. *P < 0.05 compared to noninfected cells. Pooled data of four independent experiments are shown. (c) SDF-1 secretion in the supernatant of noninfected and H. pylori-infected cells were examined by ELISA. Results correspond to the mean of three independent experiments. *P < 0.05 compared to noninfected cells
H. pylori infection upregulates CXCR4 expression on BMD-MSCs
To examine the effect of H. pylori infection on BMD-MSCs, these cells were infected with H. pylori and CXCR4 expression was measured using conventional RT-PCR, qRT-PCR, and FACS analysis. Our RT-PCR data show that CXCR4 is expressed in BMD-MSC cells and H. pylori infection significantly upregulated (3.6-fold; P < 0.035) it [Figure 4a]. When we quantified the levels of CXCR4 mRNA, we observed that H. pylori infection leads to 3.4 ± 0.9 fold upregulation of CXCR4 in these cells [Figure 4b]. CXCR4 expression was confirmed to be expressed at the protein level on the cells surface of BMD-MSCs and it was increased by H. pylori infection [Figure 4c].
Figure 4.
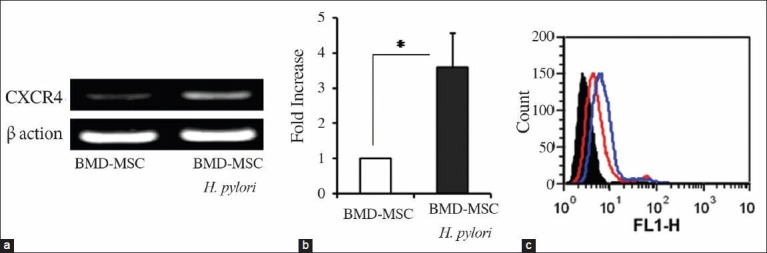
The effect of H. pylori infection on CXCR4 expression in BMD-MSCs. BMD-MSCs were infected with H. pylori for 24 h, mRNA was isolated, and CXCR4 expression was examined by qRT-PCR. (a) RT-PCR analysis of mRNA transcripts for CXCR4 in noninfected and H. pylori-infected cells. β-actin was used as internal loading control for the RT-PCR. (b) qRT-PCR shows that CXCR4 is significantly increased in H. pylori-infected cells. CXCR4 expression was normalized to an endogenous control β-actin gene. *P < 0.05 compared to noninfected cells. (c) BMD-MSCs were infected with H. pylori for 24 h, and stained with either mouse isotype IgG control or anti-human CXCR4 antibody followed by FITC conjugated goat anti-mouse antibody. CXCR4 expression was measured by FACS. Black-filled histogram shows isotype IgG control. Red and blue histograms show CXCR4 expression on the cell surface of noninfected and H. pylori-infected BMD-MSCs, respectively
Migration of BMD-MSCs toward SDF-1
To test the functionality of H. pylori-mediated CXCR4 upregulation on BMD-MSCs, we employed a chemotaxis assay and let these cells migrate toward SDF-1 gradient. As shown in Figure 5, we observed that H. pylori-treated BMD-MSCs significantly migrated (1.8-fold; P < 0.03) toward SDF-1 gradient than untreated cells, implying that upregulated CXCR4 on BMD-MSCs show a better response to SDF-1 gradient.
Figure 5.
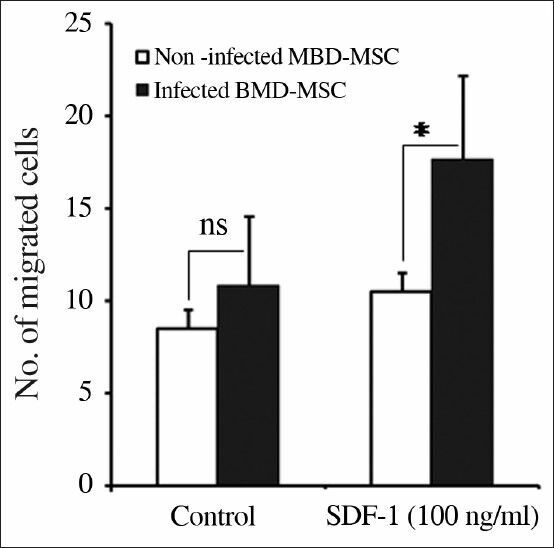
H. pylori-infected BMD-MSCs show a better response toward SDF-1 gradient. Chemotaxis of noninfected and H. pylori-infected BMD-MSC cells was evaluated toward media alone or SDF-1 (100 ng/mL). 1 × 105 cells were seeded into the upper chamber and let them to migrate into the lower chamber for 6 h. Then, the migrated cells on the lower side of the filters fixed, stained, and counted. Data of migrated cells from at least triplicate samples from three independent experiments are pooled and shown. *P < 0.05 compared to noninfected cells. ns = non-significant
DISCUSSION
Accumulating data has demonstrated that BMD-MSCs are involved in the carcinogenesis process induced in response to H. pylori infection, but the molecular mechanism of their migration to gastric mucosa has not been yet fully understood.[8] Herein, we show for the first time that H. pylori infection increases CXCR4 in BMD-MSCs and these H. pylori-treated cells show a better sense to SDF-1 gradient. In addition, H. pylori infection enhances SDF-1 secretion by gastric epithelial cells, indicating that H. pylori infection increases the communication between gastric epithelial cells and BMD-MSCs through acting on the SDF-/CXCR4 axis.
SDF-1 is a ubiquitous α-chemokine expressed by a variety of cells and has been considered as a constitutive chemokine.[26] However, many studies have postulated a putative role for SDF-1 in intestinal inflammation and experimental colitis.[27,28] Moreover, a comprehensive study conducted by Shibata et al. have demonstrated that overexpression of SDF-1 in stomach caused a significant enhancement of gastric epithelial cells proliferation, induction of inflammation, and development of tumor, particularly in combination with Helicobacter felis infection.[29] In line with this earlier study, we demonstrate in the current work that SDF-1 is expressed at both mRNA and protein level in gastric epithelial cells and H. pylori infection upregulates SDF-1 expression in these cells. By using qRT-PCR, we compared the expression of SDF-1 in gastric samples of four patients with gastric cancer who were positive for H. pylori and with four patients who were negative for H. pylori and found that the expression of SDF-1 is slightly higher (1.3-fold) in patients with H. pylori infection than those who are negative for H. pylori (data not shown). Although, the number of subjects in our study was very small there is a trend (P = 0.07) toward upregulation of SDF-1 in gastric samples from patients with H. pylori infection. Our data indicate that H. pylori infection enhances SDF-1 secretion by gastric epithelial cells and that SDF-1 might chemoattract CXCR4-expressing cells to the gastric mucosa. However, Ferrand et al. have reported that H. pylori infection was not able to upregulate SDF-1 expression in mouse intestinal cells.[30] This contrary could be explained as we used human epithelial cells which are naturally targets for H. pylori infection, but Ferrand and colleagues used mouse intestinal cell lines.
It is well known that CXCR4 is expressed on inflammatory cells and plays a vital role in trafficking of these cells to inflammatory sites.[31] In addition, CXCR4 is a key player in retention of HSCs as it expressed on these cells and mobilizing agent, granulocyte colony-stimulating factor (G-CSF) modulates the SDF-1/CXCR4 axis, leading to mobilization of HSCs from the bone marrow into the circulation.[32] We have previously reported that C5a and G-CSF significantly reduce CXCR4 expression on human neutrophils and decrease their migration toward SDF-1 gradients,[23] indicating that the SDF-1/CXCR4 axis plays a crucial role in migrating of many cells. On the other hand, as previously reported, BMD-MSCs, but not HSCs, can repopulate gastric epithelium, and progress to cancer as these cells give rise to cytokeratin-positive metaplastic cells during of chronic H. felis infection.[33] Accordingly, cytokeratin 19-expressing BMD-MSCs mobilize to the peripheral blood in response to H. felis infection and these MSCs contribute to gastric epithelial regeneration and repair.[34] Further support of this observation is obtained from the data of a recent report, which demonstrate that the SDF-1/CXCR4 axis plays a critical role in mobilization of BMD-MSCs from bone marrow to burn wounds.[35] In accordance with a previous study that demonstrated that infection of SDF-1 transgenic mice with H. felis enhances recruitment of CXCR4-positive BMD-MSCs to stomach.[29] In this study, we show that H. pylori infection increases CXCR4 expression in BMD-MSCs leading to increasing of chemotaxis of these cells toward SDF-1 gradient. On the basis of our in vitro observation, we postulate that during chronic H. pylori infection, SDF-1 is overexpressed by gastric epithelial cells and that SDF-1 could chemoattract BMD-MSCs, which possess a high level of CXCR4 expression in response to H. pylori infection, into the gastric tissue. However, we have to consider that animal studies are required to confirm our hypothesis. Furthermore, in addition to the SDF-1/CXCR4 axis there are other molecular explanations for recruitment of BMD-MSCs into the gastric epithelium. Tumor necrosis factor-alpha (TNF-α) and CCL2 have been shown to be upregulated by epithelial cells infected with H. pylori and in contrast to CCL2, TNF-α was able to induce BMD-MSC migration.[30] However, the effect of H. pylori infection on the receptors of TNF-α and CCL2 was not been examined.
In conclusion, we demonstrate that H. pylori infection upregulates both SDF-1 and CXCR4 expression in gastric epithelial cells and BMD-MSC, respectively, and that BMD-MSCs show a better response toward the SDF-1 gradient. Our data open a new window for studying migration of BMD-MSCs to gastric tissue during infection with H. pylori infection. However, more studies using animal models are required to prove this notion.
ACKNOWLEDGEMENTS
Authors thank Kurdistan University of Medical Sciences for the financial supports.
Footnotes
Source of Support: This work was supported by a grant from Kurdistan University of Medical Sciences to AJ.
Conflict of Interest: None declared.
REFERENCES
- 1.Jang BG, Kim WH. Molecular pathology of gastric carcinoma. Pathobiology. 2011;78:302–10. doi: 10.1159/000321703. [DOI] [PubMed] [Google Scholar]
- 2.Kuipers EJ. Review article: Exploring the link between Helicobacter pylori and gastric cancer. Aliment Pharmacol Ther. 1999;1:3–11. doi: 10.1046/j.1365-2036.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 3.Peek RM, Jr, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831–58. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasui W, Sentani K, Motoshita J, Nakayama H. Molecular pathobiology of gastric cancer. Scand J Surg. 2006;95:225–31. doi: 10.1177/145749690609500403. [DOI] [PubMed] [Google Scholar]
- 5.Hatakeyama M. Helicobacter pylori CagA – A bacterial intruder conspiring gastric carcinogenesis. Int J Cancer. 2006;119:1217–23. doi: 10.1002/ijc.21831. [DOI] [PubMed] [Google Scholar]
- 6.Kabir S. Effect of Helicobacter pylori eradication on incidence of gastric cancer in human and animal models: Underlying biochemical and molecular events. Helicobacter. 2009;14:159–71. doi: 10.1111/j.1523-5378.2009.00677.x. [DOI] [PubMed] [Google Scholar]
- 7.Farinati F, Cardin R, Cassaro M, Bortolami M, Nitti D, Tieppo C, et al. Helicobacter pylori, inflammation, oxidative damage and gastric cancer: A morphological, biological and molecular pathway. Eur J Cancer Prev. 2008;17:195–200. doi: 10.1097/CEJ.0b013e3282f0bff5. [DOI] [PubMed] [Google Scholar]
- 8.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–71. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson L, Stenstrom B, Chen D, Piperdi B, Levey S, Lyle S, et al. Human Barrett's adenocarcinoma of the esophagus, associated myofibroblasts, and endothelium can arise from bone marrow-derived cells after allogeneic stem cell transplant. Stem Cells Dev. 2011;20:11–7. doi: 10.1089/scd.2010.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tirode F, Laud-Duval K, Prieur A, Delorme B, Charbord P, Delattre O. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11:421–9. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Li HC, Stoicov C, Rogers AB, Houghton J. Stem cells and cancer: Evidence for bone marrow stem cells in epithelial cancers. World J Gastroenterol. 2006;12:363–71. doi: 10.3748/wjg.v12.i3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–7. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun L, Ye RD. Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin. 2012;33:342–50. doi: 10.1038/aps.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liekens S, Schols D, Hatse S. CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell mobilization. Curr Pharm Des. 2010;16:3903–20. doi: 10.2174/138161210794455003. [DOI] [PubMed] [Google Scholar]
- 15.Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- 16.Zlotnik A. New insights on the role of CXCR4 in cancer metastasis. J Pathol. 2008;215:211–3. doi: 10.1002/path.2350. [DOI] [PubMed] [Google Scholar]
- 17.Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1α-CXCR4/CXCR7 pathway inhibition: An emerging sensitizer for anticancer therapies? Clin Cancer Res. 2011;17:2074–80. doi: 10.1158/1078-0432.CCR-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, et al. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One. 2012;7:e34608. doi: 10.1371/journal.pone.0034608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cronin PA, Wang JH, Redmond HP. Hypoxia increases the metastatic ability of breast cancer cells via upregulation of CXCR4. BMC Cancer. 2010;10:225. doi: 10.1186/1471-2407-10-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazennec G, Richmond A. Chemokines and chemokine receptors: New insights into cancer-related inflammation. Trends Mol Med. 2010;16:133–44. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao C, Lu X, Bu X, Zhang N, Wang W. Involvement of tumor necrosis factor-α in the upregulation of CXCR4 expression in gastric cancer induced by Helicobacter pylori. BMC Cancer. 2010;10:1471–2407. doi: 10.1186/1471-2407-10-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 23.Jalili A, Shirvaikar N, Marquez-Curtis L, Qiu Y, Korol C, Lee H, et al. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010;38:321–32. doi: 10.1016/j.exphem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–64. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 25.Iwasa S, Yanagawa T, Fan J, Katoh R. Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric cancer is associated with lymph node and liver metastasis. Anticancer Res. 2009;29:4751–8. [PubMed] [Google Scholar]
- 26.Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia XM, Wang FY, Xu WA, Wang ZK, Liu J, Lu YK, et al. CXCR4 antagonist AMD3100 attenuates colonic damage in mice with experimental colitis. World J Gastroenterol. 2010;16:2873–80. doi: 10.3748/wjg.v16.i23.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dotan I, Werner L, Vigodman S, Weiss S, Brazowski E, Maharshak N, et al. CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm Bowel Dis. 2010;16:583–92. doi: 10.1002/ibd.21106. [DOI] [PubMed] [Google Scholar]
- 29.Shibata W, Ariyama H, Westphalen CB, Worthley DL, Muthupalani S, Asfaha S, et al. Stromal cell-derived factor-1 overexpression induces gastric dysplasia through expansion of stromal myofibroblasts and epithelial progenitors. Gut. 2013;62:192–200. doi: 10.1136/gutjnl-2011-301824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrand J, Lehours P, Schmid-Alliana A, Mégraud F, Varon C. Helicobacter pylori infection of gastrointestinal epithelial cells in vitro induces mesenchymal stem cell migration through an NF- K B-dependent pathway. PLoS One. 2011;6:e29007. doi: 10.1371/journal.pone.0029007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–24. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 33.Avital I, Moreira A, Downey RJ. The origin of epithelial neoplasms after allogeneic stem cell transplantation. Haematologica. 2006;91:ELT07. author reply ELT06. [PubMed] [Google Scholar]
- 34.Okumura T, Wang SS, Takaishi S, Tu SP, Ng V, Ericksen RE, et al. Identification of a bone marrow-derived mesenchymal progenitor cell subset that can contribute to the gastric epithelium. Lab Invest. 2009;89:1410–22. doi: 10.1038/labinvest.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu C, Yong X, Li C, Lü M, Liu D, Chen L, et al. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J Surg Res. 2013;183:427–34. doi: 10.1016/j.jss.2013.01.019. [DOI] [PubMed] [Google Scholar]


