Abstract
We investigated possible synergistic action of anticancer drug Irinotecan (IRI) combined with ethanolic (EEP) and water-soluble (WSDP) derivate of propolis on Swiss albino mice injected with Ehrlich ascites tumor (EAT). For survival analysis mice were administered WSDP and EEP (100 mg/kg) daily for 3 consecutive days, beginning on 3rd day after EAT cell (1×106) injection. IRI was administered at a dose of 50 mg/kg on days 1, 13, and 19. We simultaneously studied peripheral white blood cell count, cell types washed from the peritoneal cavity, functional activity of macrophages from peritoneal cavity, and the level of primary DNA damage in leukocytes, kidney, and liver cells using the alkaline comet assay. Three out of 9 mice per group survived the entire duration of the experiment (90 days) in groups treated with IRI combined with WSDP and EEP. All test components increased survival of mice by 7.53% to 231.54%. Combined treatment with IRI and/or WSDP and EEP significantly decreased percentage of tumor cells in the peritoneal cavity as compared to nontreated EAT-injected mice. All treated animals had significantly higher percentage of neutrophils in the peritoneal cavity in comparison to nontreated EAT-injected mice. We observed significantly higher value of DNA damage in leukocytes of mice treated with IRI and combination of IRI and/or WSDP and EEP as compared to nontreated EAT-injected mice, while the same treatment decreased DNA damage in kidney. Our results showed that addition of propolis to IRI treatment enhanced antitumor activity of IRI and prolongs survival in EAT-bearing mice, which definitely deserve further studies to clarify the possible mechanisms of antitumor actions of combined herb–drug treatments.
Key words: : Ehrlich ascites tumor, Irinotecan, propolis, survival, tumor growth
Introduction
Chemical structure and biological action of antineoplastic drug Irinotecan (IRI) is similar to naturally occurring alkaloid camptothecin from plant Camptotheca acuminata. IRI acts as topoisomerase I inhibitor, with strong antitumor activity against small cell lung cancer, nonsmall cell lung cancer, ovarian cancer, stomach cancer, breast cancer, pancreatic cancer, leukemia, lymphoma, and cervical cancer.1,2 IRI causes deleterious side-effects, such as myelosuppression, diarrhoea, nausea, vomiting, and liver dysfunction.3,4 Propolis (bee glue) has been used in medicine from ancient times in many parts of the world. It is adhesive resinous, gummy substance produced by bees. Propolis is complex mixture of more than 300 compounds, such as flavonoids, organic acids, phenols, and various kinds of enzymes, vitamins, and minerals.5 Propolis possess: strong antimicrobial effect, acting on viruses, bacteria, and fungi,6 antioxidative effect7 pronounced cytostatic, anticarcinogenic, and antitumor effect both in in vitro and in vivo tumor models,8,9 stimulative effects on hematopoietic activity and immunomodulatory effects.10–13 After its administration to mice or to human propolis does not seem to have side-effects.6
Cancer chemotherapy has been of limited success due to adverse side-effects, resistance of cancer cells, and deleterious effects to normal cells. The ethnopharmacological approach may offer valuable pharmacological leads because it is recognized that herb–drug interactions can drastically modify pharmacokinetic and pharmacodynamic properties of chemotherapy drugs.14,15
Therefore, in this study, we used water-soluble derivate of propolis (WSDP) and ethanol extract of propolis (EEP) in order to investigate their possible synergistic action with IRI on Ehrlich ascites tumor (EAT)–bearing mice by means of life span prolongation, tumor growth regression, and suppression of DNA-damaging potential of cytostatic drug in different cell types.
Materials and Methods
Animals
Swiss albino male mice bred at our conventional animal facility were used. At the start of the experiment male mice were 2 months old, with body weight of ∼20–25 g. They were housed at 20°C, with a free access to food and water. All studies were carried out according to the guidelines in force in the Republic of Croatia (Law on the Welfare of the Animals; Official Gazette 135/06 and 37/13) and in compliance to the Guide for the Care and Use of Laboratory Animals.16
Tumor cells
The Ehrlich tumor was originally described as a spontaneous murine mammary adenocarcinoma. Ehrlich tumor cells elicit a strong host immune response that makes this tumor an interesting model for analysis of the effects of drugs on tumor growth.17
EAT cells were grown in male Swiss albino mice and maintained by weekly intraperitoneal injection in an ascitis form. Mice were inoculated intraperitoneally with 1×106 viable tumor cells per each animal in a volume of 0.5 mL of 0.9% sodium chloride solution.
Irinotecan
IRI (IRI hydrochloride trihydrate) was purchased from Aventis Pharma, Dagenham, UK.
Water-soluble derivative of propolis
WSDP was prepared according to the previously described method.18 Briefly, crude propolis collected from beehives kept at north west periphery of Zagreb, Croatia, was extracted with 96% ethanol, which was filtered and evaporated to dryness in vacuum evaporator. The resultant resinous product was added to a stirred solution of 8% L-lysine (Sigma Chemie, Deisenhofen, Germany) and freeze-dried to yield the WSDP, a yellow-brown powder. Before use the WSDP was dissolved in distilled water.
Ethanolic extract of propolis
EEP was prepared by the method described by Kosalec et al.19 In brief, crude propolis (10 g) was crushed into small pieces in a mortar and mixed vigorously with 34.85 mL of 80% (V/V) ethanol during 48 hours at 37°C±1°C. After extraction, the EEP was filtered and lyophilized. Before use EEP was dissolved in ethanol and further dilutions were made in water. The final concentration of ethanol was ≤1%. Ethanol (1%) was used in the control group.
Chemical composition of propolis
“Poplar-type” propolis can be characterized by the three analytical parameters: total flavonol and flavone content, total flavanone and dihydroflavonol content, and total polyphenolic content.20 The spectrophotometric assay based on the formation of aluminum chloride complex was applied for quantification of total flavones/flavonols. For the quantification of flavanone and dihydroflavonol propolis, we used 2,4-dinitrophenylhydrazine method.21 Total polyphenolic content was measured by the Folin–Ciocalteu procedure.20 WSDP contains 2.13% of flavones and flavonols, 9.06% of flavanones and dihydroflavonols, 11.19% of total flavonoids, and 70.48% of total polyphenols. EEP contains 1.6% of flavones and flavonols, 38.60% of flavanones and dihydroflavonols, 40.20% of total flavonoids, and 84.40% of total polyphenols.19 HPLC analysis of propolis was performed according to method described by Pietta et al.22 EEP contained 2.87% of pinocembrin, 1.86% of chrysin, 0.60% of naringenin, and 1.37% of caffeic acid while WSDP contained 3.06% of pinocembrin, 2.24% of chrysin, 1.14% of naringenin, and 10.11% of caffeic acid.19,23
Experimental Design
Experiment 1
Survival analysis and tumor growth
For survival analysis experimental groups were composed of 9 mice each. Mice were administered intraperitoneally WSDP and EEP (100 mg/kg) daily for 3 consecutive days, beginning on 3rd day after EAT cell (1×106) injection. IRI was administered intraperitoneally at a dose of 50 mg/kg on days 1, 13, and 19, which simulates the repeated dosing schedules currently used clinically.3 Doses of WSDP and EEP were selected according to results of our previous studies.18,23–25 Control group that bear EAT was treated in same condition with water solution containing ethanol ≤1%.
The endpoint of experiments was determined either by spontaneous death of animals or by elective killing of animals that show signs of pain or suffer according to established criteria.16 The experiment ended on day 90.
Results are expressed as survival time range, median survival time, and the percent of mean survival time of treated animals over mean survival time of the control group (treated vs. control, T/C%). The percentage of increased lifespan (ILS%) was calculated according to the formula ILS%=(T−C)/C×100, where T represents mean survival time of treated animals and C represents mean survival time of the control group. According to the UN National Cancer Institute criteria, T/C exceeding 125% and ILS exceeding 25% indicate that the drug has significant antitumor activity.26
For tumor growth analysis mice were weighed every 4 days during the experimental period starting on day 1 after tumor injection. The growth of tumor was expressed as mean of total body weight change for each group and served as an indirect measure of tumor growth inhibition.
Experiment 2
For other methods mice were divided into groups of 5 animals each and administered intraperitoneally WSDP (100 mg/kg), EEP (100 mg/kg), and IRI 50 mg/kg daily for 3 consecutive days, beginning on 3rd day after EAT cell (1×106) injection.
Peripheral whole-blood leukocyte count
Blood samples were taken from the tail vein and processed for analysis immediately after collection. Total peripheral blood leucocytes were counted using hemocytometer.
Analysis of cell types washed from the peritoneal cavity
Mice were sacrificed in ether chamber on the 7th day after tumor cell injection. Each animal was intraperitoneally inoculated with 3 mL of saline solution prior to collection of the cells present in peritoneal cavity. We analyzed the total number of cells, differential count of the cells present in the peritoneal cavity, and functional activity of macrophages. The total number of cells present in the peritoneal cavity was counted using the standard procedure described by Oršolić et al.25 Differential count of cells present in the peritoneal cavity of mice was assigned by standard staining with May-Grünwald and a water solution of Giemsa (one part of Giemsa: two parts of water). Cell differentiation into tumor cells, macrophages, lymphocytes, and neutrophils using classic morphological patterns was conducted. Tumor cells were identified according to Fecchio et al.27 About 800 cells in each sample were differenced.
Functional activity of macrophages from the peritoneal cavity
Functional activity of macrophages from peritoneal cavity of mice was studied with spreading technique adapted from Rabinovitch and DeStefano.28 Cellular suspension was obtained from peritoneal cavity, placed on glass, and harvested at room temperature for 15 minutes. The nonadherent cells were washed out with phosphate buffer saline; the adherent cells were incubated in culture medium (RPMI-1640 and 10% fetal calf serum) and 10 nM HEPES at 37°C. Following 1 hour the culture medium was removed and cells were fixed with 2.5% glutaraldehyde. Afterward, the cells were stained with a 5% solution of Giemsa and percentage of spread cells was determined under 40×magnification on light microscopy.
Comet assay
Alkaline comet assay described by Singh et al.29 on whole-blood samples, kidney tissue, and liver tissue was performed. A comprehensive procedure is given in our previous article by Knežević et al.30 Samples were taken 1 hour after administration of the last treatment. Blood and tissue samples taken from nontreated mice were processed in parallel. Blood samples were drawn from the tail vein. Kidney and liver tissues were dissected and homogenized in ice-cold buffer (0.075 M NaCl and 0.024 M Na2EDTA; 1 g tissue/mL buffer). Two slides per animal were prepared. The slides were stained with ethidium bromide (20 μg/mL) and examined using a 250×magnification fluorescence microscope (Zeiss, Jena, Germany) equipped with an excitation filter of 515–560 nm and a barrier filter of 590 nm. A computer-based image analysis system (Comet Assay II; Perceptive Instruments Ltd., Suffolk, UK) was used. A total of 50 comets per sample per animal were scored, and data were pooled (250 comets per group). Tail intensity (% DNA in the comet tail) and tail length (expressed in micrometers) were evaluated. The values of tail length were used for the determination of the long-tailed nuclei (LTN) count as described previously.31
Statistical analysis
Kaplan–Meier method for survival analysis of treated EAT-bearing mice was employed. Log-rank test (p<0.01) was used to compare survival curves. Conventional descriptive statistics was calculated (mean±standard error, median, and range). Multiple comparisons between groups were performed on log-transformed data using the analysis of variance. LTN comet frequency statistically was evaluated using the Chi-square test. The level of statistical significance was set at p<0.05.
Statistical software package STATISTICA 7.0 (StatSoft, Tulsa, OK) was used.
Results
Effects of IRI combined with EEP and WSDP on tumor growth in EAT-bearing mice were examined by measuring survival time of treated animals during 90 days. The survival analysis showed that median survival time of all treated groups except mice treated with EEP alone, significantly increased life span as compared to nontreated EAT-injected mice (Table 1). In groups treated with IRI combined with WSDP and EEP, 3 out of 9 mice per group survived the entire duration of the experiment (90 days). All test components ILS of mice by 7.53% to 231.54% (Table 1).
Table 1.
Survival of Mice Treated with Water-Soluble Derivate of Propolis, Ethanolic Extract of Propolis, and Irinotecan
| Groupa | Survival time range (days) | Median survival time±SD (days) | ILS%b | T/NT%c | Long-term survivors (LTS)d |
|---|---|---|---|---|---|
| WSDP | 17–57 | 22.00±15.78* | 58.46 | 158.55 | 0 |
| EEP | 14–23 | 20.00±2.83 | 7.53 | 107.53 | 0 |
| IRI | 32–56 | 39.00±8.02* | 143.06 | 243.11 | 0 |
| WSDP+IRI | 31–90 | 43.50±27.37* | 230.70 | 330.69 | 3 |
| EEP+IRI | 31–90 | 52.00±27.78* | 231.54 | 331.54 | 3 |
| Nontreated EAT-injected mice | 17–19 | 17.00±0.86 | — | — | 0 |
Swiss albino mice were treated therapeutically with WSDP and EEP daily for 3 consecutive days (100 mg/kg) beginning on 3rd day after intraperitoneal injection of EAT cells (1×106). IRI was administered intraperitoneally at dose of 50 mg/kg on days 1, 13, and 19.
ILS% (increased life span %)=(T−NT)/NT×100. T, mean survival days of treated group; NT, mean survival days of nontreated EAT-injected mice.
T/NT, treated versus nontreated EAT-injected mice.
LTS, long-term survivors; mice that survive more than 90 days after treatment.
Significantly different (p<0.01) from nontreated EAT-injected mice analyzed by log-rank test.
Results are expressed as the means of each group (n=9) and are representative of three independent experiments.
EEP, ethanolic extract of propolis; WSDP, water-soluble derivate of propolis; IRI, Irinotecan; EAT, Ehrlich ascites tumor.
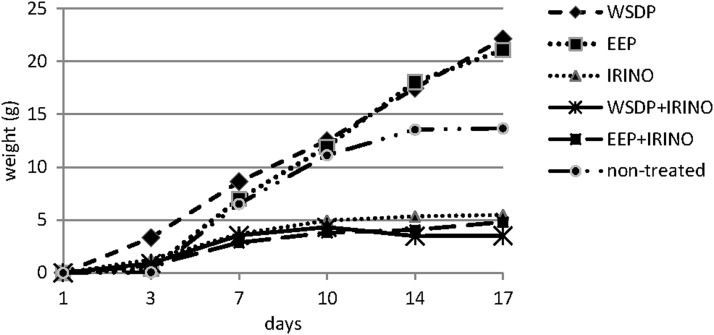
Total body weight change of mice as a measure of proliferation of EAT cells in peritoneal cavity in ascetic form are shown in Figure 1. All tested components in combined treatments with IRI decreased tumor cell proliferation as compared with IRI alone (Fig. 1).
FIG. 1.
Total body weight change of Ehrlich ascites tumor (EAT)–bearing mice treated with water-soluble derivate of propolis (WSDP) and ethanolic extract of propolis (EEP) daily for 3 consecutive days (100 mg/kg) and/or Irinotecan (IRI; 50 mg/kg) during 17 days.
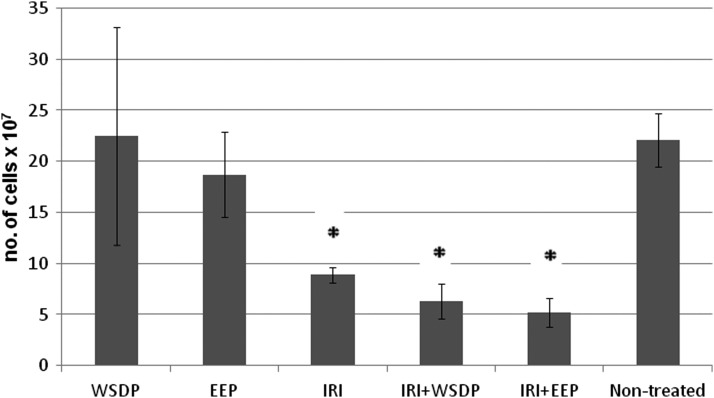
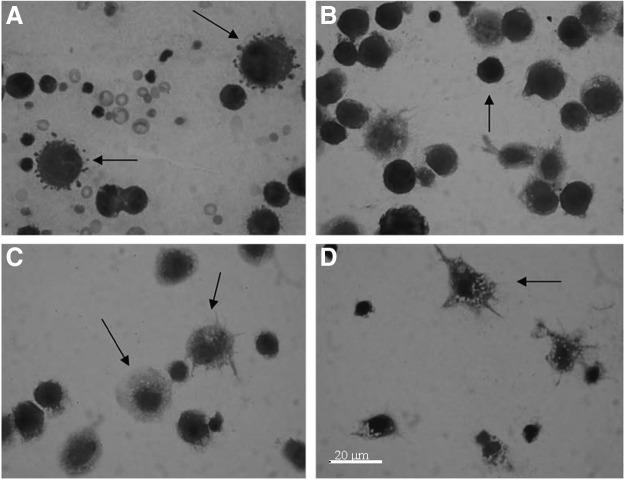
The total number of cells present in the peritoneal cavity of mice was slightly increased in the animals treated with WSDP and slightly decreased in the animals treated with EEP. Significantly decreased numbers of cells present in peritoneal cavity were observed in the animals treated with IRI alone, and in those that were simultaneously given IRI with WSDP and EEP (Fig. 2). Combined treatment with IRI and/or WSDP and EEP significantly decreased percentage of tumor cells in the peritoneal cavity as compared with nontreated EAT-injected mice (Fig. 3). On the other hand, their macrophage counts were significantly higher than in nontreated EAT-injected mice. All treated animals had significantly higher percentage of neutrophils in the peritoneal cavity in comparison with nontreated EAT-injected mice (Fig. 3). The results obtained for percentage of spreading of macrophages harvested in peritoneal cavity are shown in Figure 4. Spread cells were those that presented cytoplasmic elongation(s), while the nonspread cells were rounded (Fig. 5). Combined treatment with EEP and IRI statistically, significantly increased while treatment with IRI alone and IRI combined with WSDP significantly decreased macrophage spreading compared with nontreated EAT-injected mice (Fig. 4).
FIG. 2.
The total number of cells present in the peritoneal cavity of mice bearing EAT 7 days after EAT injection and intraperitoneal treatment with WSDP and EEP daily for 3 consecutive days (100 mg/kg) beginning on 3rd day after intraperitoneal injection of EAT cells (1×106). IRI was administered intraperitoneally at dose of 50 mg/kg daily for 3 consecutive days. *Significantly different (p<0.01) from nontreated EAT-injected mice analyzed by Student's t-test.
FIG. 3.
The percentage of cells present in the peritoneal cavity of Swiss albino mice that bear EAT after intraperitoneal treatment with WSDP and EEP daily for 3 consecutive days (100 mg/kg) beginning on 3rd day after intraperitoneal injection of EAT cells (1×106). IRI was administered intraperitoneally at dose of 50 mg/kg daily for 3 consecutive days. *Significantly different (p<0.01) from nontreated EAT-injected mice analyzed by Student's t-test.
FIG. 4.
The percentage of macrophage spreading in the peritoneal cavity of the animals on the 7th day of EAT growth after intraperitoneal treatment with WSDP, EEP, and IRI. *Statistically different compared with nontreated EAT-bearing mice (p<0.05).
FIG. 5.
Different cells from peritoneal cavity of mice treated with WSDP, EEP, and IRI. (A) Both arrows indicate apoptotic tumor cells. (B) Arrow indicates tumor cell. (C) Left arrow indicates activated macrophages with cytoplasmic vacuoles; right arrow indicates activated macrophages with elongations. (D) Arrow indicates activated macrophages with cytoplasmic vacuoles and elongations. Magnification 40×.
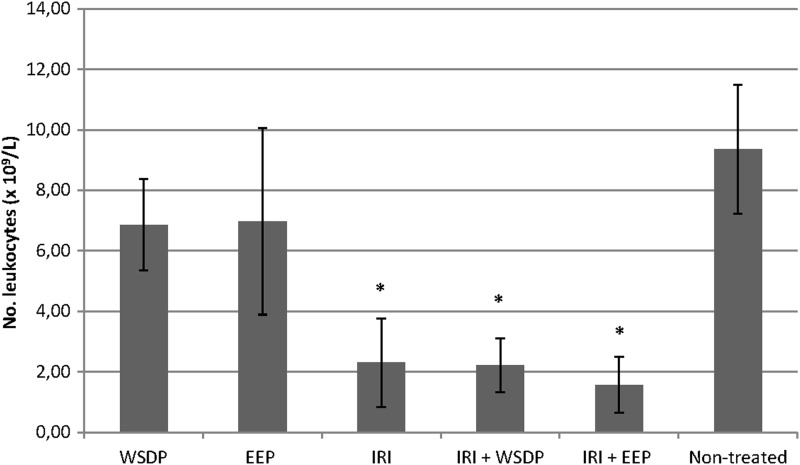
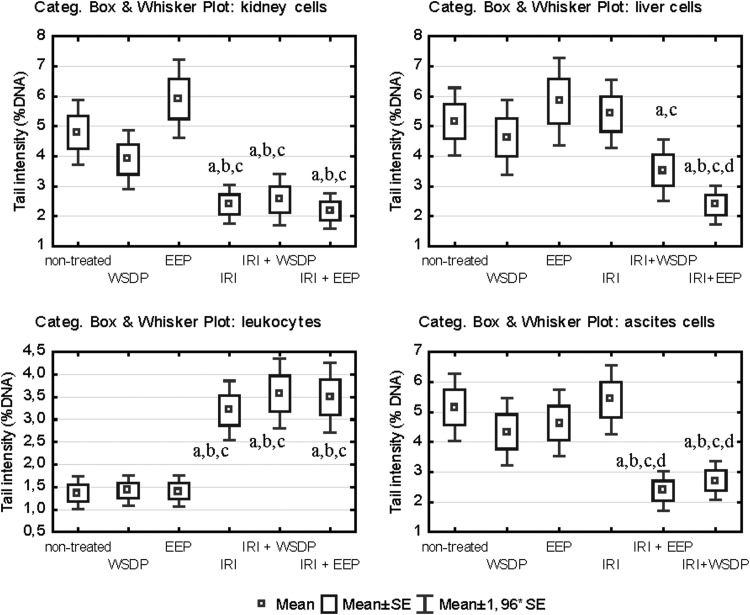
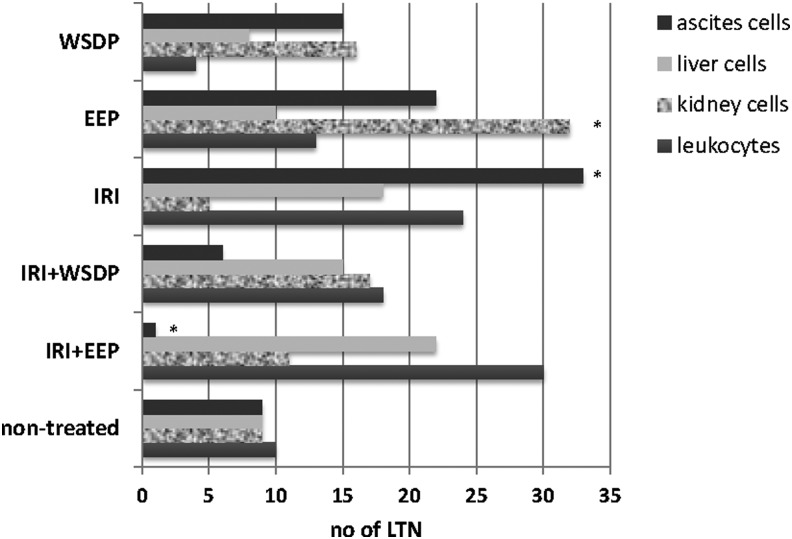
The number of leukocyte count from peripheral blood of mice treated with IRI and its combination with WSDP and EEP was significantly decreased (Fig. 6). The results of the alkaline comet assay expressed as tail intensity (% DNA) are reported in Figure 7. Single IRI and IRI combined with WSDP and EEP had the highest DNA-damaging potential in leukocytes. Interestingly, the same treatment options induced the lowest primary DNA damage in the kidney cells. Mice given a combination of IRI and WSDP or EEP had significantly lower tail intensity in their ascite cells in comparison with the values measured in all other experimental groups (Fig. 7). Figure 8 displays the values of LTN comets for all tested types of cells recorded in all experimental groups. The highest incidence of LTN comets was counted in ascite cells of mice administered with IRI as a single treatment. We found a decreased number of LTN in ascite cells, and increased number of LTN in leukocytes from mice treated with combination of IRI and EEP, as compared with nontreated EAT-injected mice. A highest incidence of LTN in kidney cells was observed in mice treated with EEP alone (Fig. 8).
FIG. 6.
Number of leukocytes in peripheral blood of therapeutically treated EAT-bearing mice. *Statistically different compared with nontreated EAT-bearing mice (p<0.05).
FIG. 7.
Results of comet assay expressed as tail intensity (%DNA) that was measured in different cell types of EAT-bearing Swiss albino mice that were given therapy with WSDP, EEP (100 mg/kg), and IRI (50 mg/kg) as single compounds or in combination. Significant differences (p<0.05) are as follows: a, as compared with nontreated EAT-injected mice; b, as compared with samples treated with WSDP; c, as compared with samples treated with EEP; d, as compared with samples treated with IRI.
FIG. 8.
Number of long-tail nucleated cells measured in different cell types of EAT-bearing Swiss albino mice that were given therapy with WSDP, EEP (100 mg/kg), and IRI (50 mg/kg) as single compounds or in combination. *Statistically different compared with nontreated EAT-bearing mice (p<0.05).
Discussion
Chemotherapy treatments are often limited with a plethora of adverse effects, particularly those related to cholinergic symptoms in the gastrointestinal tract and hematotoxicity. IRI, which was used in the present study, is often given for treatment of gastrointestinal and pulmonary malignant diseases, but its use is also restricted with broad spectrum of side-effects.1,32
It is recognized that herb–drug interactions can drastically modify pharmacokinetic and pharmacodynamic properties of chemotherapy drugs.15,33 Propolis has attracted much attention in recent years as a useful or potentially active substance in medicine and cosmetic products. The cancer protective effects of propolis and related flavonoids have been attributed to a wide variety of mechanisms, including free radical scavenging, modification of enzymes responsible for the processes of detoxification, and immunomodulation or induction of apoptosis and/or necrosis.11,34
The most important objective of this study was to evaluate possible effects of WSDP and EEP with a known antitumor drug IRI. We performed a set of experiments aimed at determining how WSDP and EEP influence the lifespan of mice and tumor growth. The most important finding of our study was that therapeutic administration of WSDP and EEP together with IRI significantly ILS of EAT-bearing mice (Table 1) and resulted in 3 out of 9 long survivors. That is in agreement with studies of Dimov et al.34 where administration of a WSDP (50 mg/kg) prevented the cyclophosphamide side-effects and enhanced the survival rate of mice. Immunomodulating activity of propolis is one of possible mechanisms that encouragements specific and nonspecific immune defense mechanism of host against neoplastic growth in experimental tumor systems.6,11 The exact mechanisms of propolis interaction with IRI are still left to be elucidated; however, substances categorized as free radical scavengers have been shown to possess antioxidant and pro-oxidant activities, influence intracellular levels of glutathione, remove important intracellular defense mechanisms against cytotoxic agents, enhance drug cytotoxicity or promote apoptosis and oxidative DNA damage in tumor cells, modulate gene expression, and influence cellular proliferation.
The results obtained by the analysis of cell types from ascitic fluid taken from peritoneal cavity indicate that mice given combined treatments (WSDP+IRI; EEP+IRI) had lower number of tumor cells and higher macrophage and neutrophil counts as compared with nontreated EAT-injected mice. It is reasonable to presume that mice surviving to the end of experiment eliminated EAT cells due to the combined activity of IRI and the beneficial effects of WSDP and EEP. Our results suggest that test components used in this study suppress the growth of EAT cells by activation of macrophages and neutrophils. It is known that functional activity of neutrophils and/or macrophages is related to the amount of reactive oxygen species (ROS). The ROS production in activated neutrophils seems to influence their lifespan, which in turn can be modulated by antioxidant properties of propolis and its constituents.35 Therefore, manipulation of these processes is likely to be a key strategy in the increase of rate or delay of apoptotic events in the complete population of circulating neutrophils and/or macrophages. Many authors suggest that antioxidants used as an adjunct in chemotherapy enhance efficacy of antineoplastic drugs and/or lower their adverse effects on surrounding normal tissue.24,33,36 It is known that standard therapy with cytostatics produces acute toxicity during treatment and that toxicity can be severe enough to cause discontinuation of therapeutic agents.33,37 Agents that could reduce the toxicity of standard cytostatic therapy on normal cells and/or that could increase the response of tumor cells to standard therapy may markedly improve the current management of human cancer. It is recognized that IRI acts as an inhibitor of the nuclear enzyme topoisomerase I, which is involved in cellular DNA replication and transcription.1,37 IRI and its active metabolite SN-38 bind to the topoisomerase-DNA complex, preventing relegation of the single-strand breaks in the DNA molecule; on the other hand, advancing replication enzymes collide with the camptothecin–topoisomerase I-DNA complex, leading to double-stranded DNA breaks.1 Based on the positive results of alkaline comet assay, we assume that the types of primary DNA damage induced in the cells of treated mice were single-strand breaks, double-strand breaks, and oxidative damage. A combination of IRI and WSDP and EEP showed the lowest levels of primary DNA damage obtained on liver, kidney, and ascitic cells as measured by the comet assay (Fig. 7). Ascitic content that served as the source of cells for the comet assay was composed predominantly of different leukocyte types and a small percentage of tumor cells (Fig. 3). This might indicate that the majority of white blood cells were not structurally or functionally impaired, and it is logical to presume that they could successfully counteract EAT. Consequently, we assume that the low number of leucocytes measured in the peripheral blood of the same experimental groups might indicate that a vast majority of cells were mobilized to the peritoneal cavity as a response to the tumor. On the other hand, a high incidence of LTN comets observed in leukocytes of mice administered single IRI and combinations with WSDP and EEP indicated that tested compounds potentiated DNA fragmentation. The results of alkaline comet assay obtained on liver cells indicate that both WSDP and EEP in combination with IRI lowered the levels of primary DNA damage. Thus, their protective capabilities were confirmed. Similar protective effects could be also seen in kidney cells of mice that were given IRI and EEP. Nevertheless, those observations have to be proved in future studies.
To conclude, results of this study showed enhanced antitumor activity of IRI combined with propolis on EAT in mice. Understanding of the molecular mechanism following the experiential effects is still limited; it appears that many different combinations of complementary modes of action may be involved. In our opinion synergistic effects of natural dietary agent and chemotherapeutic compounds definitely deserve additional studies to solve up the possible mechanisms of antitumor actions and additional useful and reliable outcome in cancer therapy.
Acknowledgments
This study was supported by the Ministry of Science, Education and Sports of the Republic of Croatia (Grant Nos. 022-0222148-2137, 119-0000000-1255, and 119-0532265-1254).
Disclosure Statement
No competing financial interests exist.
References
- 1.Hartmann JT, Lipp HP. Camptothecin and podophyllotoxin derivatives: Inhibitors of topoisomerase I and II - mechanisms of action, pharmacokinetics and toxicity profile. Drug Saf 2006;29:209. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson K. Irinotecan hydrochloride. Clin J Oncol Nurs 2002;5:179. [PubMed] [Google Scholar]
- 3.Takimoto CH, Wright J, Arbuck SG. Clinical applications of the camptothecins. Biochim Biophys Acta 1998;1400:107. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y, Villalona-Calero MA. Irinotecan: Mechanisms of tumor resistance and novel strategies for modulating its activity. Ann Oncol 2002;13:841. [DOI] [PubMed] [Google Scholar]
- 5.Bankova V. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol 2005;100:114. [DOI] [PubMed] [Google Scholar]
- 6.Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem Toxicol 1998;36:347. [DOI] [PubMed] [Google Scholar]
- 7.Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sci 2000;66:709. [DOI] [PubMed] [Google Scholar]
- 8.Sforcin JM. Cytotoxic constituents of propolis inducing anticancer effects: A review. J Pharm Pharmacol 2011;63:1378. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe MA, Amarante MK, Conti BJ, et al. Cytotoxic constituents of propolis inducing anticancer effects: A review. J Pharm Pharmacol 2011;63:1378. [DOI] [PubMed] [Google Scholar]
- 10.Constantinou A, Mehta R, Runyan C, et al. Flavonoids as DNA topoisomerase antagonists and poisons: Structure-activity relationships. J Nat Prod 1995;58:217. [DOI] [PubMed] [Google Scholar]
- 11.Sforcin JM. Propolis and the immune system: A review. J Ethnopharmacol 2007;113:1. [DOI] [PubMed] [Google Scholar]
- 12.Fresco P, Borges F, Diniz C, et al. New insights on the anticancer properties of dietary polyphenols. Med Res Rev 2006;26:747. [DOI] [PubMed] [Google Scholar]
- 13.Gupta M, Mazumder UK, Kumar RS, et al. Antitumor activity and antioxidant role of Bauhinia racemosa against Ehrlich ascites carcinoma in Swiss albino mice. Acta Pharmacol Sin 2004;25:1070. [PubMed] [Google Scholar]
- 14.Na DH, Ji HY, Park EJ, et al. Evaluation of metabolism-mediated herb-drug interactions. Arch Pharm Res 2011;34:1829. [DOI] [PubMed] [Google Scholar]
- 15.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: An updated systematic review. Drugs 2009;69:1777. [DOI] [PubMed] [Google Scholar]
- 16.Guide for the Care and Use of Laboratory Animals Official Gazette 55/13, Croatia [Google Scholar]
- 17.Sakai M, Ferraz-de-Paula V, Pinheiro ML, et al. Translocator protein (18 kDa) mediates the pro-growth effects of diazepam on Ehrlich tumor cells in vivo. Eur J Pharmacol 2010;626:131. [DOI] [PubMed] [Google Scholar]
- 18.Oršolić N, Kosalec I, Bašić I. Synergystic antitumor effect of polyphenolic components of water soluble derivative of propolis against Ehrlich ascites tumour. Biol Pharm Bul 2005;28:694. [DOI] [PubMed] [Google Scholar]
- 19.Kosalec I, Bakmaz M, Pepelnjak S, et al. Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharm 2004;54:65. [PubMed] [Google Scholar]
- 20.Popova M, Bankova V, Naydensky CH, et al. Comparative study of the biological activity of propolis from different geographic origin: A statistical approach. Mac Pharm Bull 2004;50:9 [Google Scholar]
- 21.Chang YCC, Yang MH, Wen HM, et al. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 2002;10:178 [Google Scholar]
- 22.Pietta PG, Gardana C, Pietta AM. Analytical methods for quality control of propolis. Fitoterapia 2002;73Suppl 1:S7. [DOI] [PubMed] [Google Scholar]
- 23.Benkovic V, Horvat Knezević A, Đikić D, et al. Radioprotective effects of propolis and quercetin in γ-irradiated mice evaluated by the alkaline comet assay Phytomedicine 2008;15:851. [DOI] [PubMed] [Google Scholar]
- 24.Orsolić N, Knezević AH, Šver L, et al. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J Ethnopharmacol 2004;94:307. [DOI] [PubMed] [Google Scholar]
- 25.Oršolić N, Benković V, Lisičić D, et al. Protective effects of propolis and related polyphenolic/flavonoid compounds against toxicity induced by Irinotecan. Med Oncol 2010;27:1346. [DOI] [PubMed] [Google Scholar]
- 26.Plowman J, Dykes DJ, Hollingshead M, et al.. Human tumor xenograft models in NCI drug development. In: Teicher B, ed., Anticancer Development Guide: Preclinical Screening, Clinical Trials and Approval. Totowa, NJ: Humana Press, 1997 [Google Scholar]
- 27.Fecchio D, Sirois P, Russo M, et al. Studies on inflammatory response induced by Ehrlich tumor in mice peritoneal cavity. Inflammation 1990;14:125. [DOI] [PubMed] [Google Scholar]
- 28.Rabinovitch M, DeStefano MJ. Macrophage spreading in vitro. I. Inducers of spreading. Exp Cell Res 1973;77:323. [DOI] [PubMed] [Google Scholar]
- 29.Singh NP, McCoy MT, Tice RR, et al. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 1988;75:184. [DOI] [PubMed] [Google Scholar]
- 30.Knežević AH, Dikić D, Lisičić D, et al. Synergistic effects of Irinotecan and flavonoids on Ehrlich ascites tumour-bearing mice. Basic Clin Pharmacol Toxicol 2011;109:343. [DOI] [PubMed] [Google Scholar]
- 31.Benković V, Knežević A, Đikić D, et al. Radioprotective effects of quercetin and ethanolic extract of propolis in gamma-irradiated mice. Arh Hig Rada Toksikol 2009;60:129. [DOI] [PubMed] [Google Scholar]
- 32.Kopjar N, Zeljezić D, Vrdoljak AL, et al. Irinotecan toxicity to human blood cells in vitro: Relationship between various biomarkers. Basic Clin Pharmacol Toxicol 2007;100:403. [DOI] [PubMed] [Google Scholar]
- 33.Oneschuk D, Younus J. Natural health products and cancer chemotherapy and radiation therapy. Oncol Rev 2008;1:233 [Google Scholar]
- 34.Dimov V, Ivanovska N, Manolova N, et al. Immunomodulatory action of propolis. Influence on anti-infectious protection and macrophage function. Apidologie 1991;22:155 [Google Scholar]
- 35.Suzuki I, Hayashi I, Takaki T, et al. Antitumor and anticytopenic effects of aqueous extracts of propolis in combination with chemotherapeutic agents. Cancer Biother Radiopharm 2002;17:553. [DOI] [PubMed] [Google Scholar]
- 36.Hu Z, Yang X, Ho PC, et al. Herb-drug interactions: A literature review. Drugs 2005;65:1239. [DOI] [PubMed] [Google Scholar]
- 37.Mathijssen RH, Loos WJ, Verweij J, et al. Pharmacology of topoisomerase I inhibitors Irinotecan (CPT-11) and topotecan. Curr Cancer Drug Target 2002;2:103. [DOI] [PubMed] [Google Scholar]