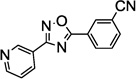
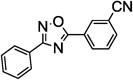
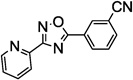
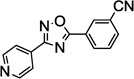
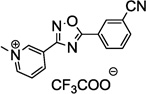
Table 1.
SAR of the 3-pyridinyl group of NS9283
PAM: Positive Allosteric Modulator for α4β2α5.
Y (for yes): 1 µM compound potentiates EC20–30 nicotine >50%;
N (for no): 1 µM compound potentiates EC20–30 nicotine <50%. Average of n = 2;
Maximum efficacy relative to NS9283.