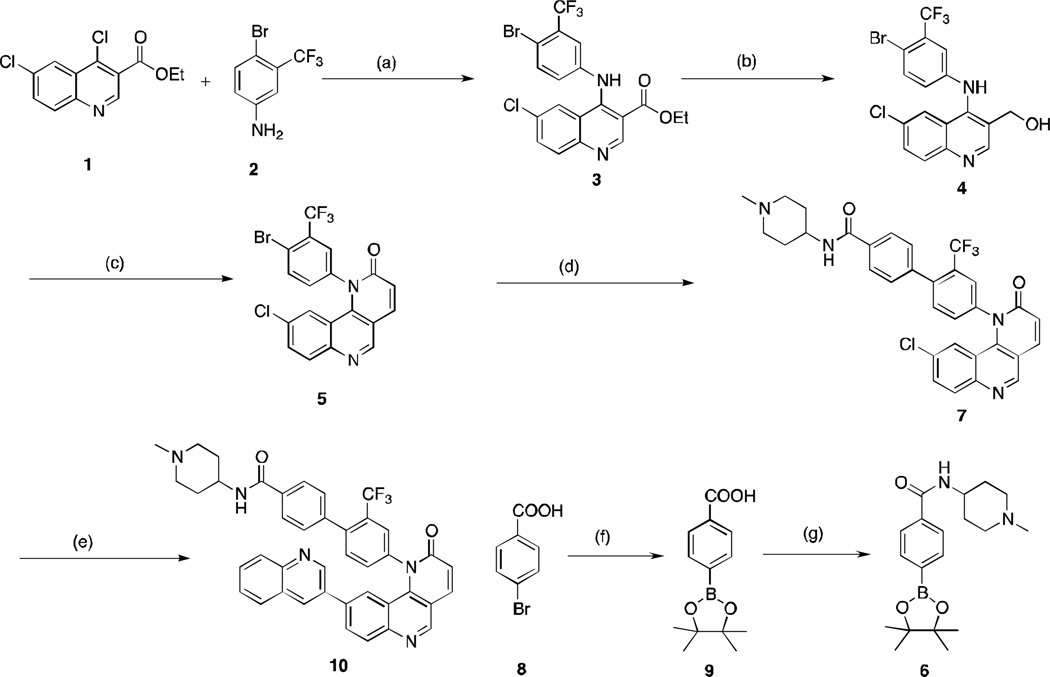
Scheme 1.
Reagents and conditions: (a) 1,4-dioxane, 85 °C, 4 h, 80% yield; (b) NaBH4, EtOH, rt, 4 h, 50% yield; (c) MnO2, CH2Cl2, rt, 2 h; then triethylphosphonoacetate, K2CO3, EtOH, 100°C, 12 h, 60% yield; (d) 6, PdCl2(Ph3P)2, Na2CO3, 1,4-dioxane, 80°C, 2 h, 60% yield; (e) quinoline-3-boronic acid, PdCl2(Ph3P)2, t-Bu-Xphos, Na2CO3, 1,4-dioxane, 100°C, 4 h, 50% yield; (f) PdCl2(dppf), bis(pinacolato)diboron, KOAc, 1,4-dioxane, 80°C, 12 h; crude material was used without purification; (g) HATU, THF, 1-methyl-piperidin-4-amine, diisopropylethylamine, rt, 50% over 2 steps.