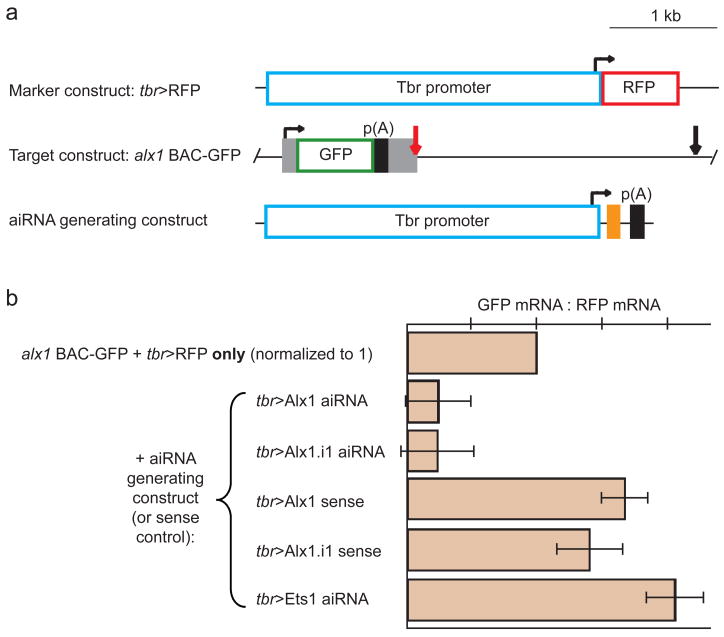
Fig. 2.
Vector designs and specificity control experiment. (A) Expression constructs coinjected in control experiments. The “marker construct” consists of a 3.5 kb tbr promoter driving expression of RFP and serves as a marker of incorporation of the concatenate of injected constructs; SV40 polyadenylation sequences, black boxes as indicated; bent arrow, start of transcription. The “target construct” is an alx BAC with a GFP coding sequence inserted by homologous recombination into the 5′UTR and containing two target sites, one spanning the junction of exon1 and intron 1 (red arrow) and the other near the middle of intron 1 (black arrow); endogenous alx1 exons, gray boxes. Five different aiRNA generating constructs were individually tested, each using the 3.5 kb tbr promoter to drive expression of a 24-bp target sequence (orange box) as specified in (B). (B) Effects of aiRNA constructs on target construct expression. In all conditions the marker and target constructs were coinjected: the ratio of GFP mRNA to RFP mRNA was then measured by real-time quantitative PCR. When an aiRNA construct targeting either the exon1/intron1 junction of the alx1 gene or an internal region of the intron was also injected, the level of GFP transcripts relative to RFP fell, since the exon1/intron1 target sequence is present in the alx1 BAC-GFP vector but not the tbr>RFP construct. The comparable sense constructs for either of these targets, meanwhile, had no effect. To assess whether aiRNA vectors could adversely effect transcript splicing, we injected a vector driving expression of a sequence antisense to the exon1/intron1 junction of ets1, which is active in the same cells as alx1. This did not decrease GFP:RFP, indicating aiRNA constructs do not lead to general defects in splicing.