Table 1.
Examples of Potential Redox Couples for Life on Past and Present-Day Mars
| Electron donor | Electron acceptor | Name | Comment |
|---|---|---|---|
| Photosynthesis | |||
| Fe2+ | Photoferrotrophy | Depends on clement surface conditions. Unlikely since the Noachian. | |
| S/S2- | Anoxygenic sulfur photosynthesis | Depends on clement surface conditions. Unlikely since the Noachian. Sulfide suggested in Curiosity data (Ming et al.,2014). Sulfur suggested at Gusev Crater (Morris et al.,2007). | |
| H2O | Oxygenic photosynthesis | Cannot be discounted on early Mars, but no atmospheric evidence for this reaction on present-day Mars. | |
| Chemolithotrophy | |||
| Fe2+ | 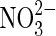 |
Anaerobic iron oxidation | Distribution of 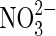 on Mars not known although fixed nitrogen is inferred (Ming et al.,2014). on Mars not known although fixed nitrogen is inferred (Ming et al.,2014). |
| Fe2+ | perchlorates | Anaerobic iron oxidation | Perchlorate can be used to oxidize iron but is not shown to be used for growth in organisms. It is included to highlight the need for investigation of perchlorate-containing redox couples. |
| H2 | CO2 | Methanogenesis, acetogenesis | Hydrogen inferred from presence of olivine and serpentine—substrates and products for H2-evolving water-rock reactions. |
| H2 | Fe3+ | Iron reduction | As above for hydrogen. |
| H2 | 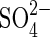 |
Sulfate reduction | As above for hydrogen. |
| H2 | oxidized nitrogen species | Distribution of oxidized nitrogen species on Mars not known. | |
| S | 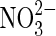 |
Sulfur oxidation | Sulfur suggested at Gusev Crater (Morris et al.,2007). |
| S | Fe3+ | Anaerobic sulfur oxidation | Occurs in acidic conditions. |
| CO | 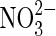 |
Anaerobic carboxydotrophy | Carbon monoxide in atmosphere. |
| Chemoorganotrophy | |||
| organics | Fe3+ | Iron reduction | The accessibility and state of organics on Mars is not known, but they are expected to arrive within carbonaceous chondrites. |
| organics | 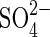 |
Sulfate reduction | As above for organics. |
| organics | 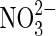 |
Nitrate reduction | As above for organics. |
| organics | perchlorate | Perchlorate reduction | As above for organics. |
| Fermentation (disproportionation) | |||
| organics | organics | Fermentation | As above for organics. |
Red denotes a half-reaction that is known to exist or has existed on Mars, green a half-reaction for which there is strong reason to suspect its presence (meteoritic organics and hydrogen). Reactions are selected from anoxic redox couples. The use of O2 as an electron acceptor for microaerophilic reactions such as hydrogen oxidation on past or present-day Mars is not explicitly ruled out. The table does not include many redox reactions that use different oxidation states of nitrogen (e.g., anaerobic ammonium oxidation with nitrite), since the fixed state of nitrogen in the martian crust is not known. Note that redox couples involving the oxidation and reduction of iron can be performed with other variable valence cations (e.g., Mn, U) that could be present in varying oxidation states in the martian crust.
