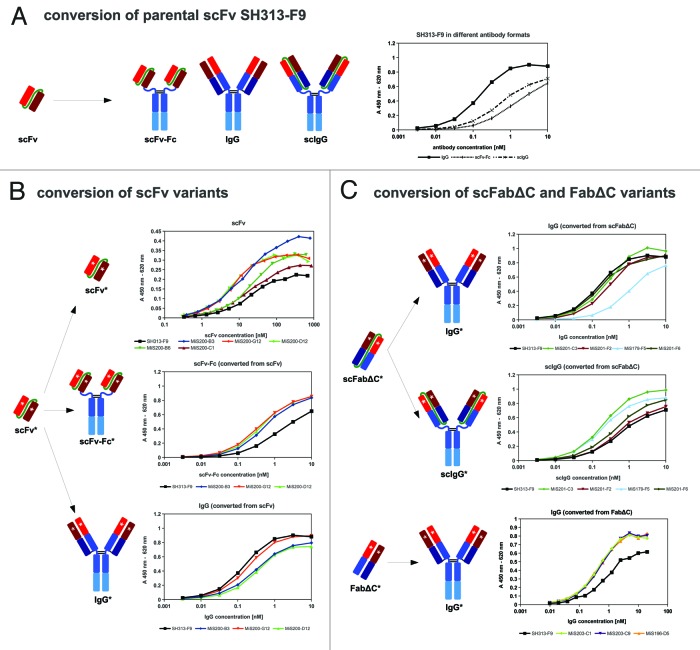
Figure 4. (A) SH313-F9 was converted into scFv-Fc, scIgG and IgG, produced in mammalian cells and tested by titration ELISA for binding to CD30. (B) Affinity maturation of SH313-F9 in the scFv format resulted in molecules with enhanced antigen binding compared with the parental scFv SH313-F9. These candidates were tested as scFv, scFv-Fc and IgG by titration ELISA for binding to CD30. (B) Affinity maturation of SH313-F9 in the scFabΔC or FabΔC formats resulted in molecules with enhanced antigen binding compared with the parental antibody. These candidates were tested as scIgG (only scFabΔC) and IgG by titration ELISA for binding to CD30. Background binding to BSA was generally very low (< 0.02) and subtracted from the shown absorption measured for CD30 binding.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.