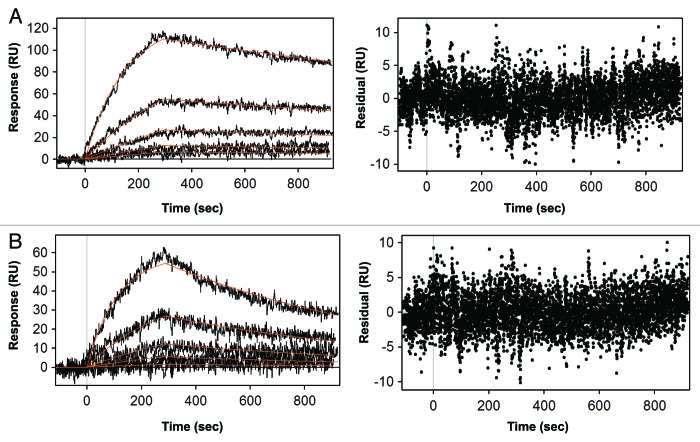
Figure 2. Quantitative kinetic analysis of XMetD binding to the hINSR. (A) Kinetic analysis of XMetD binding to the INSR solubilized from CHO-hINSR cells in the absence of insulin by SPR. (B) Kinetic analysis of XMetD binding to the INSR solubilized from CHO-hINSR cells in the presence of 1 μg/ml insulin (in running buffer) by SPR. For (A) and (B), the INSR was solubilized from CHO cells expressing the B isoform of the receptor and captured on the sensor surface via an immobilized monoclonal anti-INSR β subunit antibody (clone CT-3). XMetD concentrations ranging from 1.64‒133 nM were injected over the captured receptor to obtain association and dissociation kinetics. Residuals from the curve fit are shown adjacent to each SPR sensorgram.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.