Figure 5. CD44 associated with EphA2 on breast cancer cells.
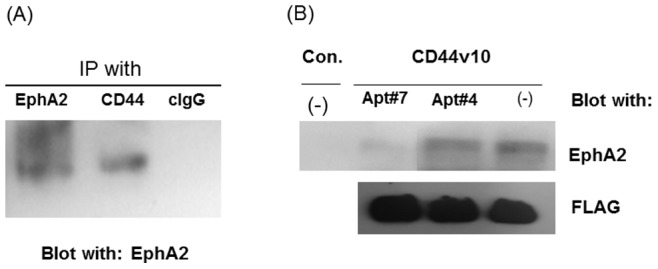
(A) HCC38 cells were lysed with 100 mM Tris-HCl (pH 7.5) containing 1% Brij35, 0.14 M NaCl, 1 mM CaCl2, 1 mM MnCl2, and a protease inhibitor cocktail by scraping and pipetting. The cell lysates were precleared and immunoprecipitated with anti-CD44 antibody clone (clone 156-3C11)-, anti-EphA2- or control IgG-protein G beads for 4 hours at 4°C. The bound proteins were released by boiling at 95°C in SDS-sample buffer under reducing conditions and separated on SDS-PAGE followed by western blotting with anti-EphA2 antibody followed by HRP-secondary antibody. The proteins are visualized with enhanced chemiluminescence (ECL) reaction. (B) Cells were harvested using 5 mM EDTA in PBS and were lysed in 100 mM Tris-HCL (pH 7.5) containing 1% Brij35, 0.14 M NaCl, 1 mM CaCl2, 1 mM MnCl2, and a protease inhibitor cocktail. The lysates were precleared with anti-FLAG (M2) agarose beads for 4 hours by shaking at 4°C. The cleared lysates were incubated with recombinant protein CD44v10 P-FLAG-anti-FLAG (M2) antibody-agarose beads (CD44v10) or anti-FLAG (M2) antibody-agarose beads (Con.) in the presence or absence of aptamers (Apt#4 and Apt#7) by shaking at 4°C for 4 hours. The beads were washed extensively with the lysis buffer. The bound proteins were released by boiling at 95°C in SDS-sample buffer under reducing conditions and separated on SDS-PAGE followed by western blotting with anti-EphA2 followed by HRP-secondary antibody or HRP-conjugated anti-FLAG (M2) antibody. The proteins are visualized with enhanced chemiluminescence (ECL) reaction. CD44 exon v10 peptide was used as a loading control for ensuring the same amount of proteins was loaded in each lane.
