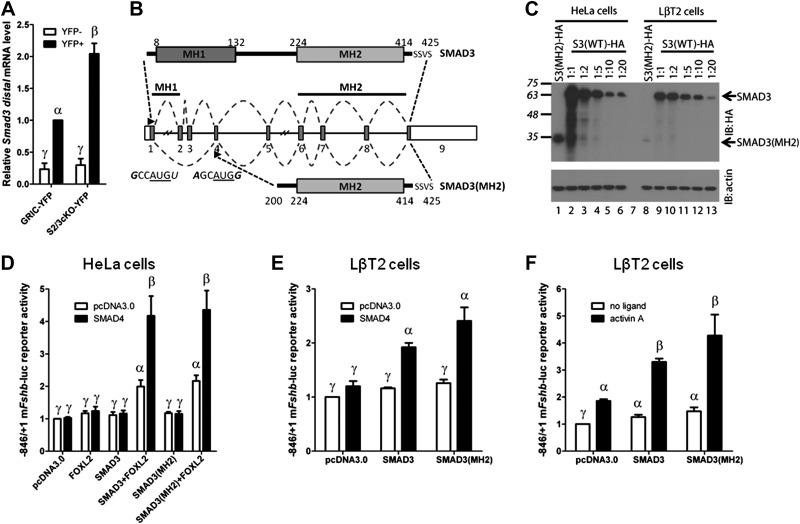
Figure 6.
Retention of a truncated Smad3 transcript coding for a functional protein in S2/3cKO mice. A) Distal Smad3 mRNA levels (forward primer in exon 8; reverse primer in exon 9), analyzed by qPCR in YFP+ and YFP− cells from the same experiment as in Fig. 1. Data represent means ± se of 3 independent sorting experiments measured in triplicate (n=3). B) Top: schematic representation of the wild-type SMAD3 protein. Amino acids are numbered. SSVS sequence at the C terminus contains the serine residues phosphorylated by the type I receptor. Middle: Smad3 gene structure with numbered exons. Splicing of the wild-type and recombined Smad3 alleles is depicted with dashed lines. Arrowheads indicate the start of translation in exon 1, and the putative alternative translation initiation site in exon 4. The nucleotide sequence surrounding the AUG sequence (underlined) of both sites is indicated. Bases that conform to the consensus Kozak sequence, −3RCCAUGG+4, at positions −3 and +4, are indicated in italics. Bottom: Representation of the truncated protein, SMAD3(MH2), arising from the recombined Smad3 allele. C) Western blot analysis of whole-cell protein lysates from HeLa (lanes 1–6) or LβT2 (lanes 8–13) cells transfected with 2 μg expression vector encoding C-terminally tagged 3X-HA tagged truncated SMAD3 (SMAD3-MH2; lane 1 and 8) or differing amounts of wild-type SMAD3-HA expression vector (lanes 2–6 and 9–13: 1:1–2 μg; 1:2-1 μg; 1:5-0.4 μg; 1:10-0.2 μg; 1:20-0.1 μg). The blot was probed with anti-HA (IB: HA, top) and anti-β-actin (IB: actin, bottom) antibodies. Arrows indicate the relative migration of full-length SMAD3 and SMAD3-MH2 products. D) HeLa cells were transfected with 225 ng/well of the −846/+1 murine Fshb-luc reporter, with 25 ng of FOXL2 and/or SMAD4 expression vectors, together with 25 ng of SMAD3 or 5 times more (125 ng) SMAD3(MH2) expression vectors. Whole-cell lysates were subjected to luciferase assays. The amount of transfected vectors was balanced across all conditions with pcDNA3.0. E) LβT2 cells were transfected with 225 ng/well of the −846/+1 murine Fshb-luc reporter, with 25 ng of SMAD4 expression vector, together with 25 ng of SMAD3 or 20 times more (500 ng) SMAD3(MH2) expression vector as indicated. The amount of transfected vectors was balanced across all conditions with pcDNA3.0. Whole-cell lysates were subjected to luciferase assays. F) LβT2 cells were transfected with 225 ng/well of the −846/+1 murine Fshb-luc reporter, along with 25 ng SMAD3 or 20 times more (500 ng) SMAD3(MH2) expression vector. The amount of transfected vectors was balanced across all conditions with pcDNA3.0. Cells were stimulated with 1 nM activin A for 24 h, or left untreated, before assaying luciferase activity. Data represent means ± se of 7 (D) or 3 (E, F) independent experiments performed in triplicate. Bars with different symbols differ significantly.