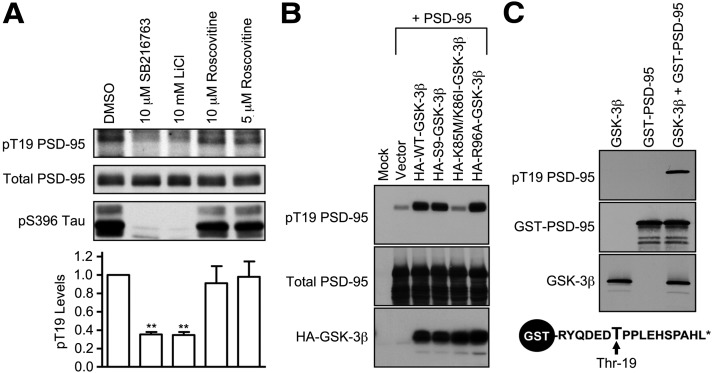
Figure 1.
Identification of GSK-3 as a PSD-95 T19 kinase. A, Cultured hippocampal neurons at DIV 25–28 were treated with DMSO, SB216763 (10 μm), LiCl (10 μm), or roscovitine (5 or 10 μm) for ∼16 h, and then immunoblotted with pT19 antibody. After stripping, the blot was reprobed with mouse total PSD-95 antibody and pS396 Tau antibody. Graph shows pT19 immunoblot intensity (corrected for total PSD-95 intensity) normalized to vehicle control. Statistical analysis was performed by one-way ANOVA, followed by Dunnett's test using DMSO treatment as control (n = 3, **p < 0.01). B, Phosphorylation of PSD-95 on T19 by GSK-3β in COS-7 cells. Wild-type PSD-95 was cotransfected with vector control (pGW1), HA-WT-GSK-3β, KD HA-KD-GSK-3β, or HA-R96A-GSK-3β. Thirty hours later, transfected COS-7 cell lysates were immunoblotted with pT19 antibody or HA antibody. After stripping, the blot was reprobed with mouse total PSD-95 antibody. C, In vitro phosphorylation of PSD-95 on T19 by recombinant purified GSK-3β. Purified GST-fused N-terminal peptide of PSD-95 (aa 13–29 of rat PSD-95) was mixed with recombinant GSK-3β. After the in vitro kinase reaction, protein samples were immunoblotted as indicated. Schematic diagram of GST-fused N-terminal peptide of PSD-95 is shown at bottom.