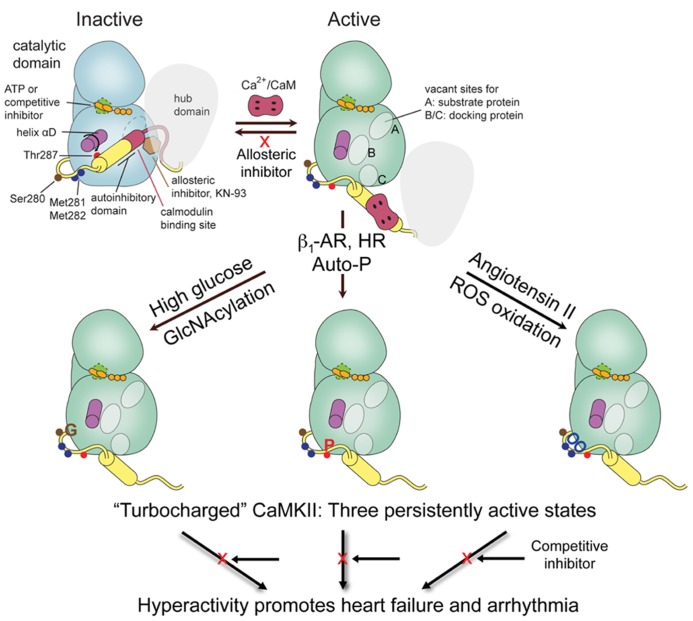
FIGURE 1.
FIGURE 1. CaMKII domain organization and schematic of activation states. One subunit of a holoenzyme composed of hub and bi-lobed catalytic domain is shown in the inactive conformation. The autoinhibitory domain or regulatory segment contains the PTM segment, important for regulation by phosphorylation, oxidation, and GlcNAcylation, followed by the CaM binding domain. Binding of Ca2+/CaM displaces the autoinhibitory domain leading to rearrangement of the lower or C-lobe of the kinase allowing substrate access to the catalytic cleft and the phosphoacceptor site A, and formation of docking sites B/C that can also be used for substrate specificity. Regulatory modifications at PTM segment by either Ca2+/CaM-stimulated autophosphorylation, e.g., by β-adrenergic stimulation or rapid heart rate (HR), oxidation by ROS, e.g., by Angiotensin II signaling, or GlcNAcylation by hyperglycemia disable the autoinhibitory domain to generate a persistently active or “turbocharged” CaMKII leading to cardiac pathophysiology.