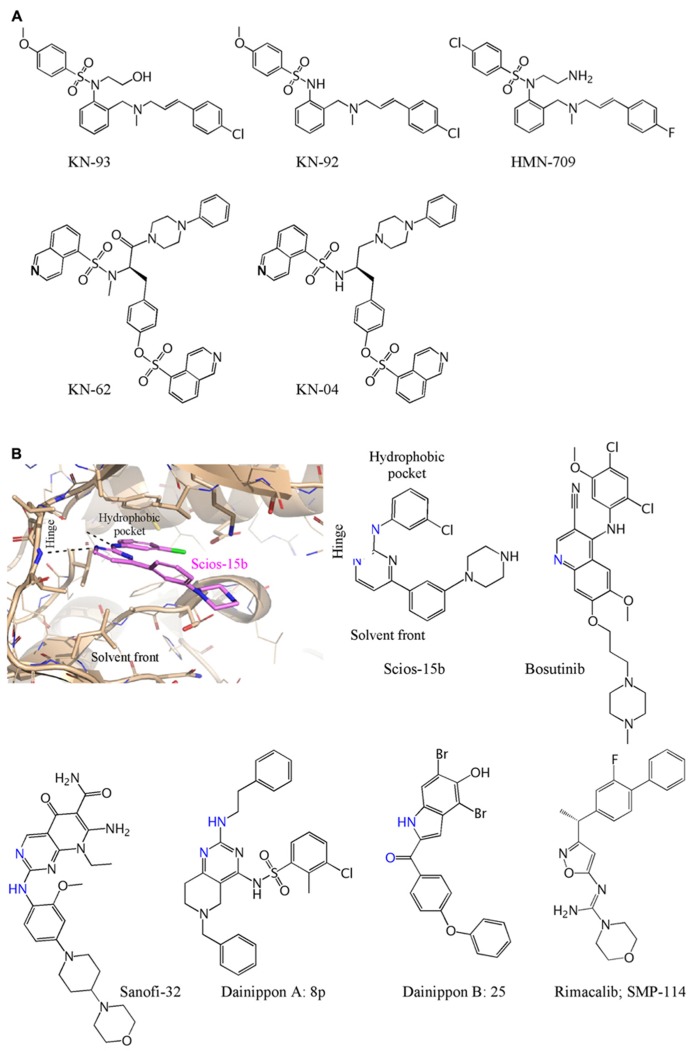
FIGURE 2.
FIGURE 2. Chemical structures of CaMKII inhibitors. (A) The ATP non-competitive inhibitors and controls are: KN-93 (Sumi et al., 1991); KN-92 (Tombes et al., 1995); KN-62 and KN-04 (Ishikawa et al., 1990) HMN-709 (Yokokura et al., 1996). (B) Computational docking of Compound 15b (Mavunkel et al., 2008) illustrates interaction of an ATP competitive inhibitor viewed from the solvent front and shown docked at the kinase “hinge” and interaction at the hydrophobic pocket. The structure of Compound 15b is shown, with the same orientation, with residues that interact at the hinge in blue based on either a crystal structures (Bosutinib) or based on modeling when a reasonable docked structure could be obtained. The compounds above are: Scios-15b (Mavunkel et al., 2008); Bosutinib (Chao et al., 2011); Sanofi-32 (Beauverger et al., 2012); Dainippon A: 8p (Asano et al., 2010); Dainippon B: 25 (Komiya et al., 2012); Rimacalib/SMP-114 (Westra et al., 2010).