Table 2.
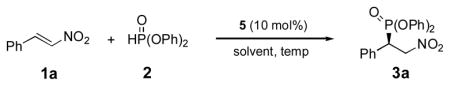
Michael addition of diphenyl phosphite (2) to trans-β-nitrostyrene (1a) catalyzed by 5[a].
| |||||
|---|---|---|---|---|---|
| entry | solvent | temp [°C] | time | conv [%][b] | ee [%] |
| 1 | toluene | rt | 1 h | 90 | 94 |
| 2 | Et2O | rt | 1 h | 81 | 93 |
| 3 | tBuOMe | rt | 1 h | 71 | 94 |
| 4 | THF | rt | 1 h | 99 | 93 |
| 5 | MeCN | rt | 1 h | 89 | 90 |
| 6 | acetone | rt | 1 h | 64 | 93 |
| 7 | CH2Cl2 | rt | 15 min | 99 | 96 |
| 8 | CH2Cl2 | 0 | 30 min | 99 (95[c]) | 97 |
| 9 | CH2Cl2 | −10 | 1 h | 99 | 98 |
| 10 | CH2Cl2 | −20 | 2 h | 99 | 98.4 |
Reactions were carried out on 0.20 mmol of 1a with 1.25 equiv of 2 and 10 mol% 5 in 1.0 mL solvent.
Reaction conversions were determined by 1H-NMR.
Isolated yield.
