Abstract
OBJECTIVES
Myocarditis is considered one of the major causes of dilated cardiomyopathy. Hepatocyte growth factor (HGF) has pleiotropic activities that promote tissue regeneration and facilitate functional improvement of injured tissue. We investigated whether the epicardial sustained-release of HGF, using gelatin hydrogel sheets, improves cardiac function in a chronic myocarditis rat model.
METHODS
Six weeks after Lewis rats were immunized with porcine cardiac myosin to establish autoimmune myocarditis, HGF- or normal saline (NS)-incorporated gelatin hydrogel sheets were applied to the epicardium (G-HGF and G-NS, respectively). At either 2 or 4 weeks after treatment, these were compared with the Control myocarditis group. Cardiac function was evaluated by echocardiography and cardiac catheterization. Development of fibrosis was determined by histological study and expression of transforming growth factor-β1 (TGF-β1). Bax and Bcl-2 levels were measured to evaluate apoptotic activity.
RESULTS
At both points, fractional shortening and end-systolic elastance were higher in the G-HGF group than in the Control and G-NS groups (P < 0.01). Fractional shortening at 2 weeks of each group were as follows: 31.0 ± 0.9%, 24.8 ± 2.7% and 48.6 ± 2.6% (Control, G-NS and G-HGF, respectively). The ratio of the fibrotic area of the myocardium was lower in the G-HGF group than in the Control and G-NS groups at 2 weeks (G-HGF, 8.8 ± 0.9%; Control, 17.5 ± 0.2%; G-NS, 15.6 ± 0.7%; P < 0.01). The ratio at 4 weeks was lower in the G-HGF group than in the G-NS group (10.9 ± 1.4% vs 18.5 ± 1.3%; P < 0.01). The mRNA expression of TGF-β1 in the G-HGF group was lower than in the Control group at 2 weeks (0.6 ± 0.1 vs 1.1 ± 0.2) and lower than that in the G-NS group at 4 weeks (0.7 ± 0.1 vs 1.3 ± 0.2). The Bax-to-Bcl-2 ratios at both points were lower in the G-HGF group than in the Control group.
CONCLUSIONS
Sustained-released HGF markedly improves cardiac function in chronic myocarditis rats. The antifibrotic and antiapoptotic actions of HGF may contribute to the improvement. HGF-incorporated gelatin hydrogel sheet can be a new therapeutic modality for myocarditis.
Keywords: Growth substances, Fibrosis, Myocarditis
INTRODUCTION
Although the aetiology of dilated cardiomyopathy (DCM) is not fully known, it is considered to be at least partly induced by autoimmune myocarditis, which is characterized by LV dilatation, systolic dysfunction, myocardial necrosis and collagen deposition [1, 2]. Despite this condition often being fatal, the number of treatments, such as heart transplantation and left ventricular assist devices (LVADs), remains limited. Moreover, because of donor shortages for heart transplantation, other treatments have long been necessary.
Hepatocyte growth factor (HGF) can be a therapeutic for myocarditis and DCM because of its cytoprotective and regenerative activities. While HGF was first found and purified from the plasma of a patient with hepatic failure, and was then molecularly cloned [3, 4], there have been several reports regarding the efficacy of HGF in cardiovascular diseases [5]. Nakamura et al. [6] reported that HGF administration improves cardiac function after ischaemia/reperfusion and that HGF exerts protective effects via its angiogenic and antiapoptotic actions. Furthermore, Taniyama et al. [7] reported that transfection of the HGF gene in the cardiomyopathic hamster model using the haemagglutinating virus of Japan (HVJ) liposome may facilitate angiogenesis and reduce fibrosis. These reports give us an idea that effective usage of HGF is a potential strategy to promote tissue regeneration and facilitate functional improvement of injured tissue in myocarditis.
As encouraging as this may seem, there are still several solutions to be found before HGF can be applied to human myocarditis and DCM, whereas a phase I/II study of patients with fulminant hepatitis or late-onset hepatic failure has already been reported [8]. First, the half-life of HGF, in solution form, is too short to maintain its biological function in situ. Secondly, gene transfer necessitates the use of viral vectors, whose expression cannot be fully controlled after administration in vivo. There have also been concerns about inflammatory responses to these vectors [9]. Thirdly, it remains unclear whether sustained delivery of exogenous HGF to failing hearts improves cardiac function in rats with autoimmune myocarditis. To establish a system for delivery, we have therefore developed HGF-incorporated gelatin hydrogel sheets, which enable HGF to be gradually released in situ over 2 weeks [10]. In experimental studies, we demonstrated that application of gelatin hydrogel sheets can improve ventricular contractility and can attenuate fibrosis in both spontaneously hypertensive and myocardial infarction rat models [11, 12]. However, the effects of HGF gelatin hydrogel sheets on autoimmune myocarditis have not been explored in depth.
Our hypothesis was that epicardial sustained-release of HGF, using the gelatin hydrogel sheets, improves cardiac function in a rat autoimmune myocarditis model through its antifibrotic and antiapoptotic effects. Experimental autoimmune myocarditis was produced in the myocardium of Lewis rats by immunization with cardiac myosin [1, 2]. This model has demonstrated that active myocarditis subsides at 6 weeks after immunization, while post-myocarditic DCM develops in the chronic phase [1]. Previous studies have reported that about one-third of subjects die from extensive myocardial necrosis [2].
METHODS
Rat autoimmune myocarditis model
All experimental procedures were conducted in accordance with Kyoto University's guidelines for animal care and the ‘Guide for the Care and Use of Laboratory Animals’, published by the National Institutes of Health.
Five-week old male Lewis rats (weighing 120–160 g; Japan SLC, Inc., Hamamatsu, Japan) were used. The DCM model was produced by means of induction of autoimmune myocarditis [13]. In brief, 1 mg/0.1 ml of purified cardiac myosin from a porcine heart was mixed with an equal volume of Freund's complete adjuvant (Difco; BD Diagnostic Systems, Sparks, MD, USA) and injected into a footpad. Six weeks after immunization, these rats served as a model of heart failure owing to autoimmune myocarditis.
Echocardiography
Rats were anaesthetized with 1% isoflurane at 6 weeks after autoimmunization. Harvard-type ventilators were used for respiratory control. Left ventricular (LV) function was evaluated by a Vivid 7 echocardiography machine with an 11-MHz phased array transducer (GE Medical, Milwaukee, WI, USA). Echocardiographic measurements were performed as described previously [13, 14]. Briefly, after the rats were anaesthetized, their chests were shaved and they were placed in the supine position on a table. A two-dimensional targeted M-mode echocardiogram was obtained and averaged along the short-axis view of the LV at the level of the papillary muscles over three consecutive cardiac cycles according to the American Society of Echocardiography leading-edge method. The following parameters were measured three times and averaged by M-mode tracing: LV internal end-diastolic and end-systolic dimension (LVIDd, LVIDs) and diastolic and systolic wall thickness (LVWTd, LVWTs). Values were calculated using the following equation:
Preparation and application of gelatin hydrogel sheets
Gelatin was isolated from bovine bone collagen by an alkaline process using calcium hydroxide (gelatin; Nitta Gelatin Co, Osaka, Japan). Gelatin hydrogel sheets were prepared as described previously [10]. Briefly, after mixing 100 µl of 25 wt% glutaraldehyde aqueous solution with 50 ml of 5 wt% gelatin aqueous solution at 40°C, the mixture was cast into a polypropylene tray and left for 12 h at 4°C to perform chemical cross-linking of gelatin. The resulting hydrogel sheet was then punched out and immersed in 100 mM glycine aqueous solution at 37°C for 1 h. The cross-linked gelatin hydrogel sheet was twice washed with double-distilled water, freeze-dried and sterilized with ethylene oxide gas. Square sheets (20 × 20 mm) were impregnated with an aqueous solution containing 100 µg of human recombinant HGF (courtesy of Prof Tsubouchi, Kagoshima University). HGF-incorporated gelatin hydrogel sheets can gradually release HGF in situ over 2 weeks [10, 11].
Six weeks after immunization, fractional shortening was measured by echocardiography. The hearts of normal Lewis rats (age, 11 weeks) served as control to confirm the development of myocarditis. A small pericardial incision was made through a left-sided thoracotomy under general anaesthesia with 1% isoflurane. Without sutures, gelatin hydrogel sheets with saline or HGF were attached to the epicardium of the entire LV free wall. The pericardium was closed with interrupted polypropylene sutures. We confirmed that the sheet stayed on the LV epicardium during the entire study period by means of serial echocardiography.
Study groups
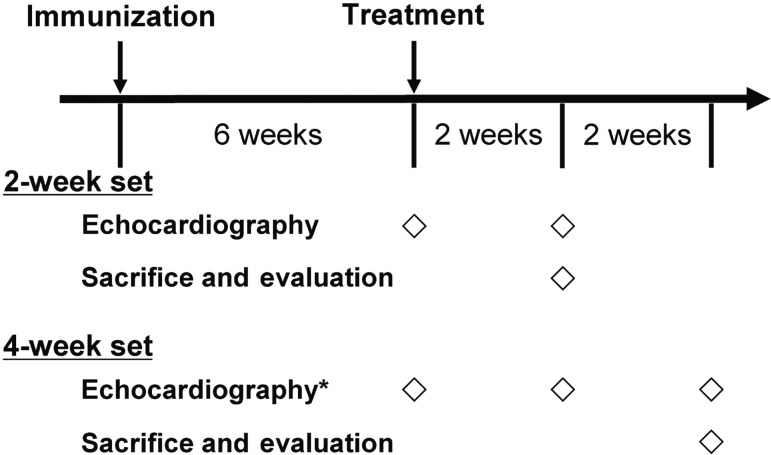
After echocardiographic examination, the rats in which myocarditis developed with an LV fractional shortening <40% were then randomly divided into the following three groups: (i) the Control group, which had the myocarditis heart without application of gelatin hydrogel sheets or HGF (n = 14), (ii) the gelatin-normal saline (G-NS) group, which had the myocarditis heart with application of gelatin hydrogel sheets and 100 µl of saline (n = 14) and (iii) the gelatin-HGF (G-HGF) group (n = 14), which had the myocarditis heart with application of gelatin hydrogel sheets and 100 µg of HGF. The concentration of HGF was determined based on our previous study, where 40 µg of HGF alone did not exert a favourable effect on cardiac function [12]. The hearts in each group were evaluated at either 2 or 4 weeks after treatment (establishing a 2-week set and a 4-week set; Fig. 1). The timing of the evaluation was chosen following our previous studies [11, 12] and based on the duration of the HGF release from the gelatin hydrogel sheets as described above.
Figure 1:
Protocol. *Echocardiography was serially performed only in the 4-week set (G-NS and G-HGF groups). G-HGF: gelatin-hepatocyte growth factor; G-NS: gelatin-normal saline.
Physiological studies
Echocardiography and cardiac catheterization were carried out under general anaesthesia with 1% isoflurane. LV pressure–volume loop analysis was performed as described elsewhere [15]. Following echocardiography, the right carotid artery was cannulated with a pressure–volume catheter (SPR-869, Millar Instruments, Houston, TX, USA) that was advanced into the aorta and then into the left ventricle. The inferior vena cava (IVC) was exposed via midline laparotomy. Following the measurement of the baseline pressure–volume loops, a series of loops was recorded after LV preload was reduced by directly compressing IVC. End-systolic elastance was determined from these pressure–volume loops (>5 loops) using the Integral 3 system (Unique Medical, Tokyo, Japan). Time constant (τ) was measured from baseline loops.
In order to follow the time course of LV geometric changes in the rats from the 4-week set, echocardiographic evaluation was performed both 2 and 4 weeks after treatment in the G-NS and G-HGF groups (7 rats in each group; Fig. 1).
Histology
Following catheterization, each heart was removed after animals were sacrificed with carbon dioxide; the LV myocardium was transversely sliced into sections (2 mm in diameter) at the base of the papillary muscle and then fixed in 10% buffered formalin. The remaining LV myocardium was frozen at −80°C until analysis. Transverse sections of the LV myocardium were stained with Sirius-red reagents thus determining the fibrotic area. The Sirius-red-stained myocardium was serially photographed with high-power field, and a whole section was reconstructed from the serial images using a microscope (BIOREVO BZ-9000; Keyence Corp., Osaka, Japan). Red-stained fibrotic area was automatically calculated with an automated image analysis system (BIOREVO BZ-9000). The stained area was calculated as a percentage of the total area excluding the left and right ventricular cavities.
Analysis of messenger RNA expression
Total messenger RNA (mRNA) was prepared from the frozen LV pieces where gelatin hydrogel sheets were applied (n = 7, in each group) with TRIzol reagent (Life Technologies Corporation, Carlsbad, CA, USA), and reverse transcribed with the SuperScript III first-strand synthesis system (Invitrogen). Quantitative reverse-transcription polymerase chain reaction was performed using a TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA, USA) and amplified with the StepOnePlus system (Applied Biosystems). Polymerase chain reaction conditions included 40 cycles of denaturing at 94°C for 20 s and primer annealing/extension at 62°C for 60 s. The polymerase chain reaction sequence of transforming growth factor-β1 (TGF-β1) was reported in our previous research [16]. The TaqMan rodent glyceraldehyde-3-phosphate dehydrogenase control reagent was used to detect rat glyceraldehyde-3-phosphate dehydrogenase as the internal standard. In each sample, the expression level of the target gene was normalized against glyceraldehyde-3-phosphate dehydrogenase levels.
Measurement of apoptosis-related proteins
Bax and Bcl-2 levels in tissues where gelatin hydrogel sheets were applied (n = 7 per group) were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Uscn Life Science, Inc., Wuhan, China). ELISA procedures were carried out according to the manufacturer's protocol. Results are reported as a Bax-to-Bcl-2 ratio.
Statistical analysis
All data are presented as means ± standard error of the mean. Comparisons between two groups were made using unpaired or paired t-tests. Differences among three groups were evaluated using a one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. All statistical analyses were performed with IBM SPSS Statistics version 19 (IBM, Armonk, NY, USA). Statistical significance was set at the level of P < 0.05.
RESULTS
Development of autoimmune myocarditis
Autoimmune myocarditis developed in 42 of the 85 rats at 6 weeks after autoimmunization. Twenty-four rats died before treatment, while the remaining 19 rats with fractional shortening of >40 were excluded from the study. No significant differences were observed in the baseline echocardiographic parameters among the study groups (Table 1), although fractional shortening in the study groups was significantly lower than that in normal rats.
Table 1:
Baseline echocardiographic data
| Control | G-NS | G-HGF | P-value** | Normal rats | |
|---|---|---|---|---|---|
| Body weight (g) | 304.6 ± 4.9* | 296.4 ± 4.3* | 306.1 ± 4.2* | 0.27 | 339.6 ± 3.0 |
| LVIDd (mm) | 7.94 ± 0.13* | 8.26 ± 0.21* | 7.87 ± 0.12* | 0.19 | 5.26 ± 0.19 |
| LVIDs (mm) | 5.23 ± 0.16* | 5.76 ± 0.27* | 5.30 ± 0.20* | 0.17 | 2.41 ± 0.16 |
| Systolic thickening | 1.58 ± 0.08 | 1.49 ± 0.09 | 1.48 ± 0.07 | 0.65 | 1.45 ± 0.04 |
| Fractional shortening (%) | 34.2 ± 1.7* | 30.6 ± 1.7* | 32.9 ± 1.5* | 0.30 | 54.6 ± 1.9 |
*P < 0.01: difference compared with the normal Lewis rats of the same age. **P-values: differences between the Control, G-NS and G-HGF groups by one-way ANOVA.
G-HGF: gelatin-hepatocyte growth factor; G-NS: gelatin-normal saline; LVIDd: left ventricular internal end-diastolic dimension; LVIDs: left ventricular internal end-systolic dimension.
Effects of HGF on cardiac function
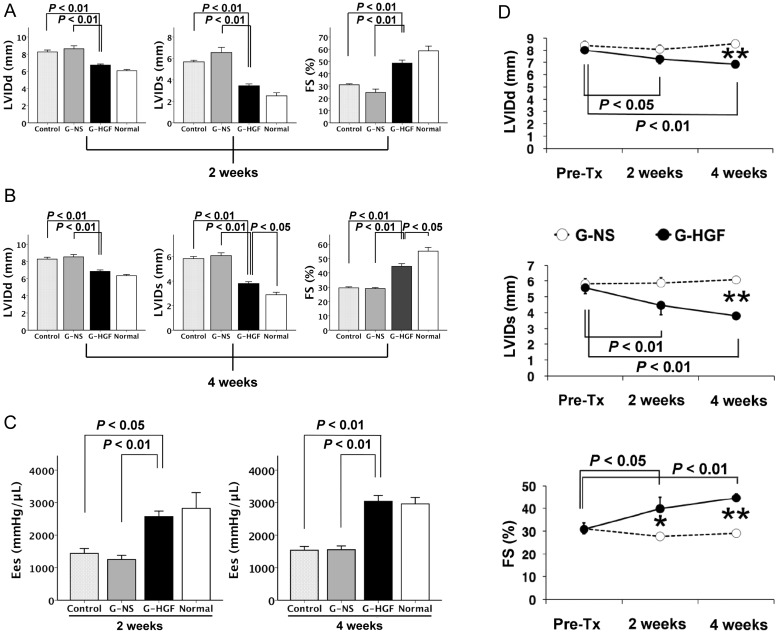
No rats died or showed a pyrogenic response after surgery. Echocardiography and cardiac catheterization showed that, at both 2 and 4 weeks after surgery, and when compared with the G-HGF group, the other two groups had significant deterioration of LV systolic functionality characterized by enlargement of the LV cavity and both had decreased LV fractional shortening and end-systolic elastance (Fig. 2A–C). However, there were no significant differences, regarding these variables, between the Control and the G-NS groups. Among the three groups, no significant differences were observed in the other echocardiography and cardiac catheterization parameters (Table 2).
Figure 2:
(A) Echocardiographic studies of the 2-week set. (B) Echocardiographic studies of the 4-week set. (C) End-systolic elastance (Ees) analysis at 2 and 4 weeks after treatment. All values are represented as means ± SEM. (D) The time course of left ventricular geometric changes in the 4-week set (G-NS and G-HGF groups). *P < 0.05, **P < 0.01: difference between G-NS (open circles) and G-HGF (filled circles). FS: fractional shortening; G-HGF: gelatin-hepatocyte growth factor; G-NS: gelatin-normal saline; LVIDd: left ventricular internal end-diastolic dimension; LVIDs: left ventricular internal end-systolic dimension; Pre-Tx: pretreatment.
Table 2:
Physiological studies
| Two weeks |
Four weeks |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | G-NS | G-HGF | Normala | Control | G-NS | G-HGF | Normala | |
| Systolic thickening | 1.5 ± 0.1 | 1.3 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 |
| Tau (ms) | 9.3 ± 0.8 | 10.4 ± 0.5 | 11.2 ± 1.2 | 9.6 ± 1.2 | 9.5 ± 0.3 | 9.5 ± 0.5 | 10.7 ± 0.5 | 11.8 ± 1.8 |
aNormal: normal Lewis rats of the same age.
G-HGF: gelatin-hepatocyte growth factor; G-NS: gelatin-normal saline.
Figure 2D depicts the time course of LV geometric changes in the rats from the 4-week set. Although LVIDd and LVIDs in the G-NS group were similar during the 4-week period, those in the G-HGF group had decreased significantly. Accordingly, LV fractional shortening at 2 and 4 weeks after surgery was significantly higher in the HGF group than in the G-NS group (P < 0.05, P < 0.01 in unpaired t-tests, respectively). However, in the G-HGF group, no significant differences in LVIDd, LVIDs and fractional shortening was observed between 2 and 4 weeks after treatment (P = 0.29, P = 0.27, P = 0.37 in paired t-tests, respectively).
Histology
When we assessed myocardial fibrosis using Sirius-red-stained sections (Fig. 3), we found significantly greater fibrosis in the Control and G-NS groups than in the G-HGF group 2 weeks after treatment (17.5 ± 0.2%, 15.6 ± 0.7%, 8.8 ± 0.9%, respectively; P < 0.01, P < 0.01, respectively). At 4 weeks after surgery, the fibrotic area of the G-HGF group was significantly lower than that of the G-NS group (10.9 ± 1.4%, 18.5 ± 1.3%, respectively; P < 0.01). There were no significant differences, regarding myocardial fibrosis, between the Control and the G-NS groups at both time points.
Figure 3:
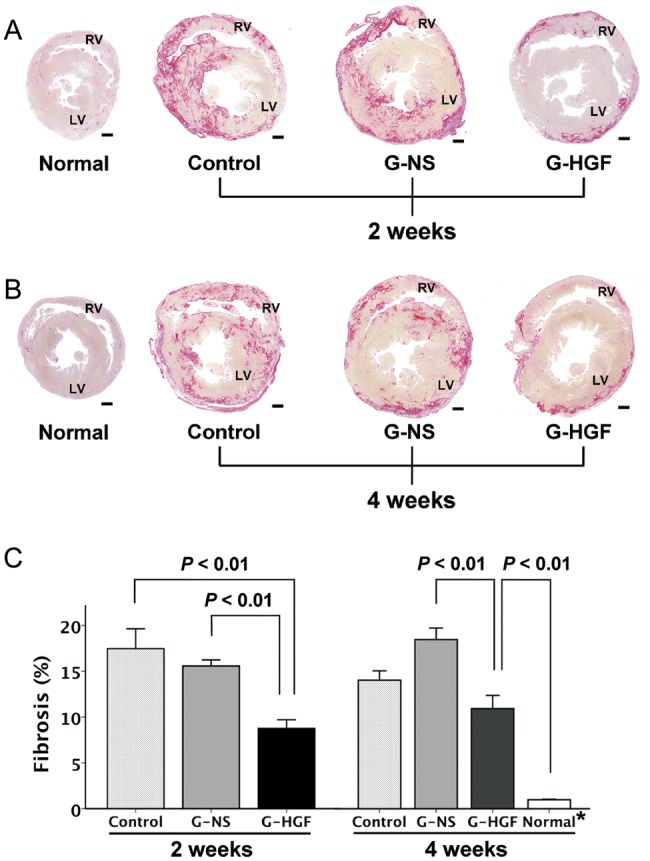
(A) Sirius-red-stained sections at 2 weeks after treatment. (B) Sirius-red-stained sections at 4 weeks after treatment. (C) Quantitative analysis of fibrotic area as a percentage. Bars = 1 mm. All values are expressed as means ± SEM. G-HGF: gelatin-hepatocyte growth factor; G-NS: gelatin-normal saline; LV: left ventricle; RV: right ventricle.
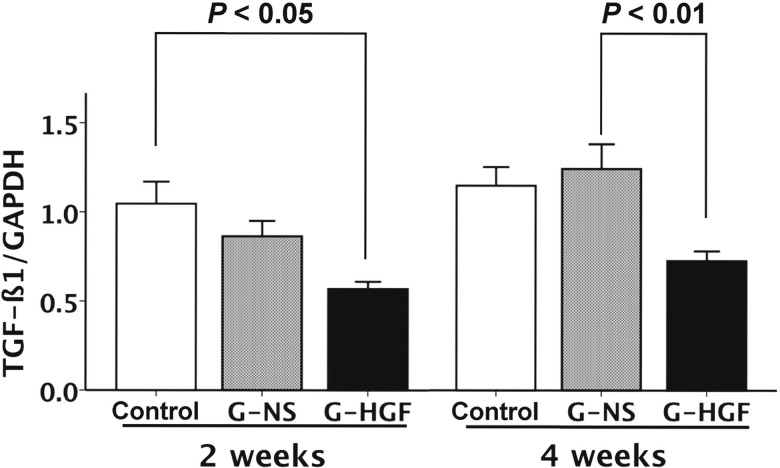
Analysis of mRNA expression
The mRNA expression of TGF-β1 was higher in the Control group than in the G-HGF group 2 weeks after treatment (P = 0.01; Fig. 4). Similarly, the mRNA expression of TGF-β1 was higher in the G-NS group than in the G-HGF group 4 weeks after treatment (P < 0.01; Fig. 4).
Figure 4:
mRNA expression of TGF-β1. G-HGF: gelatin-hepatocyte growth factor; G-NS: gelatin-normal saline; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; TGF-β1: transforming growth factor-β1.
Measurement of apoptosis-related proteins
In order to test whether HGF modulates apoptosis, ELISA for Bax and Bcl-2 was performed. The Bax-to-Bcl-2 ratios at both 2 and 4 weeks after treatment were lower in the G-HGF group than in the Control group (P = 0.04 and < 0.01, respectively; Fig. 5). The ratio at 4 weeks after surgery was lower in the G-HGF group than in the G-NS group (P = 0.03; Fig. 5).
Figure 5:
Apoptosis-related proteins; Bax-to-Bcl-2 ratios. G-HGF: gelatin-hepatocyte growth factor; G-NS: gelatin-normal saline.
DISCUSSION
Main findings
This study demonstrated that sustained-release of HGF using gelatin hydrogel sheets improves myocardial contractility in a rat myocarditis model. There were no significant differences in LV dimensions or systolic function between the Control and the G-NS groups. Therefore, placement of the saline-incorporated hydrogel sheet did not affect cardiac function when compared with the Control group. Histological study revealed that HGF administration attenuated the ratios of the fibrotic area. Evaluation of TGF-β1 mRNA expression was consistent with this result. The proapoptotic-to-antiapoptotic protein ratios were lower after the application of HGF gelatin hydrogel sheets than in the Control and the G-NS groups.
Delivery of HGF
In order to address the problem of the short HGF half-life in solution form, investigators either administered daily subcutaneous injections of HGF for 3 weeks [17] or used the transfection of HGF genes by using adenovirus or HVJ [18, 19]. However, there have been concerns about possible adverse effects of HGF associated with systemic administration of HGF, and inflammatory responses to these vectors [9].
In order to establish a delivery system, we have developed HGF-incorporated gelatin hydrogel sheets, which enable HGF to be gradually released in situ over 2 weeks. We previously followed tissue concentrations of HGF up to 4 weeks after epicardial application of an HGF-incorporating gelatin hydrogel sheet to using 125I-labeled HGF as described elsewhere [10, 11]. In brief, HGF levels remaining in the myocardium were 101 ± 8 and 21 ± 2 ng/g at 1 week and 2 weeks, respectively, but were below detectable levels thereafter. The present study provides breakthrough evidence that the HGF gelatin hydrogel sheet preserves LV systolic function in a rat myocarditis model.
Although gene transfer with viral vectors showed good results [18], gene expression cannot be fully controlled after transfection. In connection to this, there have been concerns about inflammatory responses to these vectors [9]. In contrast, our delivery system can easily control the release of HGF by changing the water content of the gelatin hydrogel [10]. Gelatin of protein is degraded by proteolysis. The higher the glutaraldehyde concentration used for hydrogel preparation, the higher the crosslinking extent of hydrogels. Higher extent of crosslinking may result in less susceptibility to proteolysis. Moreover, the gelatin hydrogel sheet is fully degraded in the body, thus circumventing inflammatory or pharmacological responses in vivo [11]. Considering that our method necessitates a single application of the HGF gelatin hydrogel sheet, it is more feasible than multiple injection of HGF from a clinical point of view [17, 20]. When HGF is administered systemically in its solution form in vivo, it rapidly diffuses into the circulation and disappears 1 day after the injection [10, 11]. Frequent injection of HGF solution at physiologically excessive doses may be necessary to induce the biological effects to be expected. We have to consider that persistent or prolonged circulation of HGF could be even harmful (e.g. neoplasms). In contrast, we previously confirmed that the blood HGF level stayed under the limit of detection for 2 weeks after the first day when applied with the gelatin hydrogel sheets [11].
One of the limitations is that this technique requires a major surgical intervention (i.e. thoracotomy). However, we believe that it might be clinically applicable to DCM or myocarditis patients in conjunction with other surgical interventions (e.g. LV volume reduction surgery) or LVAD. Considering clinical utility of the gelatin hydrogel sheets, minimally invasive surgical procedures (e.g. video-assisted thoracotomy) may also be useful.
DCM model
The DCM model in this study was induced by autoimmune myocarditis and is characterized by LV dilatation, systolic dysfunction, myocardial necrosis and collagen deposition [1, 2]. Lewis rats that were immunized with porcine cardiac myosin develop acute myocarditis that starts ∼2 weeks from the induction [2]. The inflammation and necrosis subsides within 6 weeks, while fibrotic area gradually increases between the 3rd and 6th weeks. This model has been demonstrated to progress into the state similar to DCM in the chronic phase [1, 2, 13]. There have been several animal models simulating human DCM: Coxsackievirus-infected mice [20], cardiomyopathic Syrian hamster (BIO TO-2) [7, 17, 19], doxorubicin-injected mice [18] and stroke-prone spontaneously hypertensive rats [11]. We adopted the autoimmune myocarditis model because it has been suggested that it closely resembles the fulminant form of human myocarditis and the DCM from autoimmune myocarditis at its chronic phase. As given in Table 1 and Fig. 3A and B, LV dimensions increased and fractional shortening decreased when compared with those of normal Lewis rats of the same age.
Myocardial contractility
We evaluated end-systolic elastance as an indicator of myocardial contractility by means of a conductance- and pressure-measuring catheter. This methodology has some advantages over other approaches. First, it is unaffected by loading conditions and heart rate. Secondly, end-systolic elastance reflects not only contractility but also chamber end-systolic stiffness: fibrosis [15].
Cardiac function was evaluated at both 2 and 4 weeks in the rats in the 4-week set that were sacrificed at 4 weeks after surgery. Serial echocardiographic study showed that cardiac contractility had already improved at 2 weeks after treatment, when compared with pretreatment. No differences were observed between 2 and 4 weeks for LVIDd, LVIDs or fractional shortening. We previously reported HGF levels in the myocardium after implantation of the HGF gelatin hydrogel sheet [11]. Levels gradually decreased over time thereafter. These two findings suggest that 2 weeks may be long enough for this method of HGF delivery to exert its effects. However, further studies are required to test how long the contractile recovery lasts after application of the HGF gelatin hydrogel sheet.
Fibrotic area and TGF-β1
This study showed that the fibrotic area in the G-HGF group was lower than in the Control and G-NS groups. It is possible that HGF exerts its beneficial effects on cardiac function, at least in part, through antifibrotic action. Myocardial fibrosis is related to the number of abnormal clinical features and the extent of LV systolic dysfunction in DCM. This may also represent an alternative prognostic marker for a functional decline. Therefore, HGF is expected to be a potential therapeutic option for clinical myocarditis and DCM.
TGF-β1 is known to be a key factor for promotion of tissue fibrosis. Taniyama et al. [7] reported that tissue fibrosis is regulated by a balance between TGF-β1 and HGF production. In this study, analysis of the mRNA expression demonstrated that HGF application suppressed the TGF-β1 gene expression, which in turn supports the results of the histological study. Given the chronic phase of autoimmune myocarditis, HGF may exert its antifibrotic actions not only by inhibition of collagen synthesis through suppression of TGF-β1 gene expression but degradation of collagen through activation of matrix metalloproteinase-1 [7]. Nakamura et al. [17] showed suppression of TGF-β1, type I collagen and ANP expression by exogenous HGF, which was consistent with the improvement of established myocardial fibrosis and hypertrophy in the late stage of the cardiomyopathic hamster model. In addition, Futamatsu et al. [21] demonstrated that HGF gene therapy was effective in attenuating established inflammation through its effects on T-cell mediated immunity. They also revealed that HGF gene transfer on the same day as the immunization inhibits the development of autoimmune myocarditis. In combination with our results, HGF therapy might be effective in both early and chronic phases of the autoimmune myocarditis. Reduced fibrosis may lead to increased blood flow in the myocardium, which contributes to the improvement of global systolic function.
This method of HGF administration can be an adjunct therapy in human DCM or myocarditis cases. We previously reported that the ratio of the fibrotic area and apoptosis increases with time in rat hearts with DCM, under mechanical unloading after heterotopic transplantation, although myocardial contractility was preserved [22]. HGF gelatin hydrogel sheets may thus offset the drawbacks of mechanical unloading.
Apoptosis
Nakamura et al. [6, 17] reported that subcutaneous administration of HGF decreased the number of terminal dUTP nick end-labeling (TUNEL)-positive cardiomyocytes in the ischaemia/reperfusion injury rat model and the cardiomyopathic hamster model. Futamatsu et al. [21] also reported that the HFG gene therapy resulted in a reduction in the incidence of apoptotic cardiomyocytes in the same experimental autoimmune myocarditis model as ours. On the other hand, it was reported that TUNEL-positive cells were only rarely detected in the doxorubicin-induced cardiomyopathy model [18]. We analysed whether HGF would alter the balance of apoptosis-related proteins by using ELISA. Bax is known to be proapoptotic, whereas Bcl-2 has an antiapoptotic effect [23, 24]. ELISA analysis showed that the Bax-to-Bcl-2 ratios at both 2 and 4 weeks after treatment were lower in the treatment group (Fig. 5). The balance of Bax and Bcl-2 was found to be antiapoptotic along with the suppressed gene expression of TGF-β1, which induces hypertrophy and apoptotic cell death in cardiomyocytes [17]. Based on this observation, attenuated apoptosis may be another mechanism by which the HGF gelatin hydrogel sheet preserved cardiac function in the DCM rat model. However, further evaluations using other examination methods for apoptotic parameters (e.g. TUNEL and caspase) are necessary.
Limitations
The present study has several limitations. First, DCM model in this study was produced by inducing autoimmune myocarditis. This animal model may not exactly correspond with idiopathic cardiomyopathy; however, it has been used and presented elsewhere [13, 22]. Secondly, although the amount of 100 µg was sufficient for HGF to exert its positive effects on cardiac function in the myocarditis rat model, the optimal or safe amount of HGF and its release profile have not been determined. Thirdly, because human myocardium is thicker than that in rats, it is unclear whether tissue HGF concentrations in the myocardium obtained by the same delivery system are sufficiently high for HGF to exert its activities. Fourthly, we did not evaluate the adverse effects of HGF. All rats in the G-NS and G-HGF groups survived during the study period. In addition, tissue HGF levels in the myocardium, blood, lungs and liver were evaluated, and no side-effects were observed in our previous study using HGF gelatin hydrogel sheets in a hypertensive rat model [11]. Other investigators have also reported no side-effects in their lung model [25]. Nevertheless, longer observation periods are necessary to rule out neoplastic changes after HGF application. Finally, although the present findings regarding attenuation of fibrosis and suppressed gene expression of TGF-β1 explain, at least in part, therapeutic effects of HGF, other biological activities of HGF may explain additional mechanisms (e.g. angiogenesis and anti-inflammation) responsible for improvement of deteriorated cardiac function.
CONCLUSIONS
Sustained-release of HGF, using gelatin hydrogel sheets, improves LV systolic function in a rat myocarditis model. The beneficial effects are attributable to the antifibrotic action of HGF. HGF favourably alters expression of fibrosis-related mRNA in vivo. It is also possible that the antiapoptotic action of HGF may contribute the preservation of myocardial contractility. HGF-incorporated gelatin hydrogel sheet can be a new therapeutic modality for chronic myocarditis.
Acknowledgements
We thank Ms Kataoka for her technical assistance with histological sample preparation.
Conflict of interest: none declared.
REFERENCES
- 1.Hirono S, Islam MO, Nakazawa M, Yoshida Y, Kodama M, Shibata A, et al. Expression of inducible nitric oxide synthase in rat experimental autoimmune myocarditis with special reference to changes in cardiac hemodynamics. Circ Res. 1997;80:11–20. doi: 10.1161/01.res.80.1.11. [DOI] [PubMed] [Google Scholar]
- 2.Kodama M, Hanawa H, Saeki M, Hosono H, Inomata T, Suzuki K, et al. Rat dilated cardiomyopathy after autoimmune giant cell myocarditis. Circ Res. 1994;75:278–84. doi: 10.1161/01.res.75.2.278. [DOI] [PubMed] [Google Scholar]
- 3.Gohda E, Tsubouchi H, Nakayama H, Hirono S, Sakiyama O, Takahashi K, et al. Purification and partial characterization of hepatocyte growth factor from plasma of a patient with fulminant hepatic failure. J Clin Invest. 1988;81:414–9. doi: 10.1172/JCI113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyazawa K, Tsubouchi H, Naka D, Takahashi K, Okigaki M, Arakaki N, et al. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem Biophys Res Commun. 1989;163:967–73. doi: 10.1016/0006-291x(89)92316-4. [DOI] [PubMed] [Google Scholar]
- 5.Hiramine K, Sata N, Ido A, Kamimura R, Setoyama K, Arai K, et al. Hepatocyte growth factor improves the survival of rats with pulmonary arterial hypertension via the amelioration of pulmonary hemodynamics. Int J Mol Med. 2011;27:497–502. doi: 10.3892/ijmm.2011.616. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Mizuno S, Matsumoto K, Sawa Y, Matsuda H. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J Clin Invest. 2000;106:1511–9. doi: 10.1172/JCI10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taniyama Y, Morishita R, Aoki M, Hiraoka K, Yamasaki K, Hashiya N, et al. Angiogenesis and antifibrotic action by hepatocyte growth factor in cardiomyopathy. Hypertension. 2002;40:47–53. doi: 10.1161/01.hyp.0000020755.56955.bf. [DOI] [PubMed] [Google Scholar]
- 8.Ido A, Moriuchi A, Numata M, Murayama T, Teramukai S, Marusawa H, et al. Safety and pharmacokinetics of recombinant human hepatocyte growth factor (rh-HGF) in patients with fulminant hepatitis: a phase I/II clinical trial, following preclinical studies to ensure safety. J Transl Med. 2011;9:55. doi: 10.1186/1479-5876-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary. Circulation. 2000;102:E73–86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 10.Ozeki M, Ishii T, Hirano Y, Tabata Y. Controlled release of hepatocyte growth factor from gelatin hydrogels based on hydrogel degradation. J Drug Target. 2001;9:461–71. doi: 10.3109/10611860108998780. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi G, Tambara K, Sakakibara Y, Ozeki M, Yamamoto M, Premaratne G, et al. Control-released hepatocyte growth factor prevents the progression of heart failure in stroke-prone spontaneously hypertensive rats. Ann Thorac Surg. 2005;79:1627–34. doi: 10.1016/j.athoracsur.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 12.Tambara K, Premaratne GU, Sakaguchi G, Kanemitsu N, Lin X, Nakajima H, et al. Administration of control-released hepatocyte growth factor enhances the efficacy of skeletal myoblast transplantation in rat infarcted hearts by greatly increasing both quantity and quality of the graft. Circulation. 2005;112(9 Suppl):I129–34. doi: 10.1161/CIRCULATIONAHA.104.526293. [DOI] [PubMed] [Google Scholar]
- 13.Horii T, Tambara K, Nishimura K, Suma H, Komeda M. Residual fibrosis affects a long-term result of left ventricular volume reduction surgery for dilated cardiomyopathy in a rat experimental study. Eur J Cardiothorac Surg. 2004;26:1174–9. doi: 10.1016/j.ejcts.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Nishina T, Nishimura K, Yuasa S, Miwa S, Nomoto T, Sakakibara Y, et al. Initial effects of the left ventricular repair by plication may not last long in a rat ischemic cardiomyopathy model. Circulation. 2001;104(12 Suppl 1):I241–5. doi: 10.1161/hc37t1.094522. [DOI] [PubMed] [Google Scholar]
- 15.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–34. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuneyoshi H, Nishina T, Nomoto T, Kanemitsu H, Kawakami R, Unimonh O, et al. Atrial natriuretic peptide helps prevent late remodeling after left ventricular aneurysm repair. Circulation. 2004;110(11 Suppl 1):II174–9. doi: 10.1161/01.CIR.0000138348.77856.ef. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, Matsumoto K, Mizuno S, Sawa Y, Matsuda H. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. Am J Physiol Heart Circ Physiol. 2005;288:H2131–9. doi: 10.1152/ajpheart.01239.2003. [DOI] [PubMed] [Google Scholar]
- 18.Esaki M, Takemura G, Kosai K, Takahashi T, Miyata S, Li L, et al. Treatment with an adenoviral vector encoding hepatocyte growth factor mitigates established cardiac dysfunction in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;294:H1048–57. doi: 10.1152/ajpheart.01102.2007. [DOI] [PubMed] [Google Scholar]
- 19.Kondoh H, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Kitagawa-Sakakida S, et al. Combined strategy using myoblasts and hepatocyte growth factor in dilated cardiomyopathic hamsters. Ann Thorac Surg. 2007;84:134–41. doi: 10.1016/j.athoracsur.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 20.Tang QZ, Shen DF, Huang ZR, Xiong R, Wu H, Huang J, et al. Potential role of N-cadherin in hepatocyte growth factor (HGF) mediated improvement of the cardiac function of dilated cardiomyopathy mice. Int J Cardiol. 2008;127:442–3. doi: 10.1016/j.ijcard.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Futamatsu H, Suzuki J, Mizuno S, Koga N, Adachi S, Kosuge H, et al. Hepatocyte growth factor ameliorates the progression of experimental autoimmune myocarditis: a potential role for induction of T helper 2 cytokines. Circ Res. 2005;96:823–30. doi: 10.1161/01.RES.0000163016.52653.2e. [DOI] [PubMed] [Google Scholar]
- 22.Muranaka H, Marui A, Tsukashita M, Wang J, Nakano J, Ikeda T, et al. Prolonged mechanical unloading preserves myocardial contractility but impairs relaxation in rat heart of dilated cardiomyopathy accompanied by myocardial stiffness and apoptosis. J Thorac Cardiovasc Surg. 2010;140:916–22. doi: 10.1016/j.jtcvs.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Nakagami H, Morishita R, Yamamoto K, Taniyama Y, Aoki M, Yamasaki K, et al. Hepatocyte growth factor prevents endothelial cell death through inhibition of bax translocation from cytosol to mitochondrial membrane. Diabetes. 2002;51:2604–11. doi: 10.2337/diabetes.51.8.2604. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee S, Stewart AS, Bish LT, Jayasankar V, Kim EM, Pirolli T, et al. Viral gene transfer of the antiapoptotic factor Bcl-2 protects against chronic postischemic heart failure. Circulation. 2002;106(12 Suppl 1):I212–7. [PubMed] [Google Scholar]
- 25.Ono M, Sawa Y, Matsumoto K, Nakamura T, Kaneda Y, Matsuda H. In vivo gene transfection with hepatocyte growth factor via the pulmonary artery induces angiogenesis in the rat lung. Circulation. 2002;106(12 Suppl 1):I264–9. [PubMed] [Google Scholar]