Figure 1.
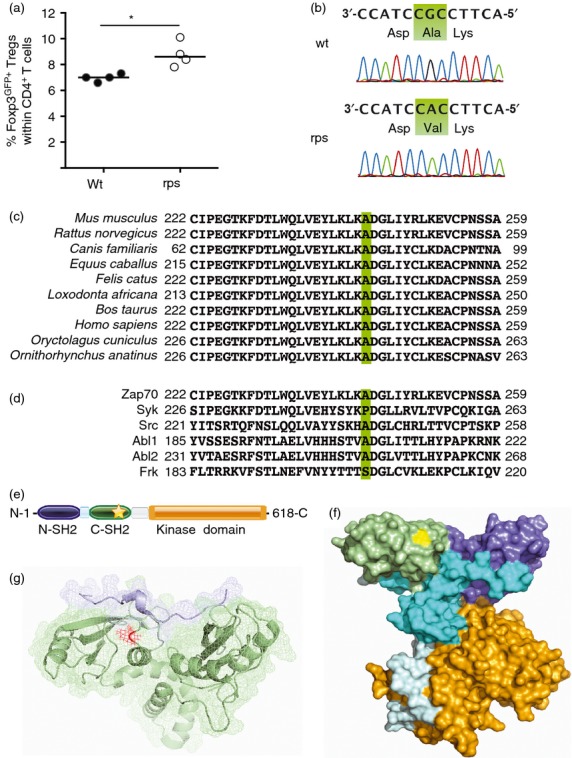
Increased peripheral regulatory T (Treg) cell frequencies in Zap70rps mice with an A243V in the C-terminal SH2-domain of Zap70. (a) Peripheral blood lymphocytes from wild-type and rps mutant mice were assessed for the percentage of Foxp3GFP+ Treg cells within the CD4+ T-cell compartment at 5–6 weeks of age. (b) Sanger sequencing of Zap70 in wild-type and rps mutant mice confirmed a C to T mutation at location 36778405, resulting in an alanine to valine change at amino acid 243. (c) Conservation of the rps mutation site between the mouse, rat, dog, horse, cat, elephant, bovine, human, rabbit and platypus homologous mammal sequences. (d) Partial conservation of the rps mutation site between related murine kinases, Zap-70, Syk, Src, Abl1, Abl2 and Frk. (e) Location of the A243V amino acid change (star) within the domain structure of Zap70. Identified are the N-terminal SH2 domain (purple), interdomain A (blue), C-terminal SH2 domain (green), interdomain B (grey) and kinase domain (orange). (f) Location of the A243 residue (yellow) within the cloud structure of Zap70. Identified are the N-terminal SH2 domain (purple), interdomain A (blue), C-terminal SH2 domain (green), interdomain B (grey) and kinase domain (orange). (g) Modelling of SH2 domains (green) in contact with TCR-ζ peptide (blue). The A243 residue is shown in red at the binding interface. Structures from PyMOL. *P < 0·05.
