Abstract
Introduction
The largest randomized controlled trial that compared the efficacy of carotid endarterectomy (CEA) with carotid artery stenting (CAS) showed equivalent outcomes for the composite end point of postoperative stroke, myocardial infarction (MI), or death. However, CAS had a higher risk of postoperative stroke, and CEA had a higher risk of MI. We hypothesize that there is a differential association of postoperative stroke, compared with that of postoperative MI, with reduced long-term survival after carotid revascularization when compared with neither complication.
Methods
The Vascular Study Group of New England database was used to identify all CEA and CAS procedures performed between 2003 and 2011. Patients were stratified according to whether they experienced an in-hospital postoperative stroke (minor or major), MI (troponin elevation, electrocardiographic changes, or clinical symptoms), or neither. Primary study end point was survival during the first year and the first 5 years postoperatively. Multivariable Cox proportional hazards models compared the magnitude of association of stroke and MI on survival.
Results
Of 8315 patients, 81 (0.97%) experienced postoperative MI, and 63 (0.76%) experienced stroke. During the first year after operation, survival significantly differed among the three groups: neither, 96%; MI, 84%; stroke, 77% (log-rank P < .0001). After adjusting for confounders, survival after postoperative stroke (hazard ratio [HR], 6.6; 95% confidence interval [CI], 3.7–12; P < .0001) was nearly twofold less than that after postoperative MI (HR, 3.6; 95% CI, 2–6.8; P < .0001). During the first 5 years postoperatively, multivariable modeling showed postoperative stroke and postoperative MI remained independent predictors of decreased survival, but the magnitude of association was similar (HR, 2.7; 95% CI, 1.7–4.3 [P < .0001] vs HR, 2.8; 95% CI, 1.8–4.3 [P < .0001]).
Conclusions
During the first year after operation, postoperative stroke conferred a twofold lower survival than that after postoperative MI. By 5 years after operation, these survival curves converged, and the survival disadvantage associated with stroke became similar to that of MI. These data suggest that different postoperative complications after carotid revascularization have different implications for patients, with decreased short-term survival in patients experiencing a postoperative stroke. These findings help to inform our interpretation of studies that have used a composite end point in order to evaluate the comparative effectiveness of revascularization strategies.
The optimal treatment modality for carotid artery stenosis remains controversial. Surgical options include carotid endarterectomy (CEA) and carotid artery stenting (CAS), but several trials have demonstrated conflicting outcomes.1–6 The Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S)1 and Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy (SPACE)7 trials failed to demonstrate noninferiority of CAS for the composite end point of stroke and death. The Carotid Revascularization Endarterectomy vs Stent Trial (CREST) trial reported no significant difference for the composite end point of postoperative stroke, myocardial infarction (MI), or death.3 However, subgroup analyses of individual end points in CREST demonstrated a higher risk of stroke after CAS, and conversely, a higher risk of MI after CEA.
Despite the conflicting results among the trials, these studies had a decisive effect on practice patterns at the national level, as evidenced by a doubling of use of CAS in 2005 after the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial, and then in 2007, decreased use of CAS after the publication of EVA-3S and SPACE.8–10 At the national level, this demonstrates a willingness by practitioners to change in response to evidence but also a lack of consensus on what that evidence suggests.
Some of these conflicting results likely arise from the use of composite primary end points. Efforts to tease out differences in outcomes have relied on subgroup analyses, which inherently lack statistical power and have the potential for biases of multiple testing.
In this context, the relative effect of a postoperative MI compared with that of a postoperative stroke on long-term survival becomes critical to the interpretation of studies evaluating the comparative effectiveness of alternative carotid revascularization strategies. We hypothesize that there is a differential association of postoperative stroke, compared with postoperative MI, on long-term survival after carotid revascularization.
METHODS
Study design
Prospectively collected data from the Vascular Study Group of New England (VSGNE) database was retrospectively reviewed to identify all patients who underwent CAS or CEA between January 1, 2003, and December 31, 2011; the unit of analysis was the index carotid revascularization. The VSGNE is a regional cooperative quality-improvement initiative, developed in 2002, to prospectively evaluate outcomes in patients undergoing vascular surgical procedures. It consists of 140 physicians from 25 academic and community medical centers across New England.
Carotid revascularization was performed by a surgeon, or in the case of CAS, an interventionalist, and eight such interventionalists contributed data to this cohort. Details of this registry have been published previously11 and are available online (www.vsgne.org). Data are physician-reported at the time of operation and include preoperative, intraoperative, and in-hospital postoperative details. Follow-up data are entered at ~1 year postoperatively. All information is sent to a central data repository where it is aggregated and audited. Trained nurses or clinical data abstractors enter data; research analysts are blinded to patient, surgeon, and hospital identity. Since the inception of the database, a claims-based audit system has been used to ensure >95% capture of consecutive operations performed at each center.
Study exclusion criteria included patients who underwent combined CEA and coronary artery bypass grafting (CABG), patients who experienced a postoperative MI and a postoperative stroke, and patients who were missing postoperative MI or stroke data. Although patients who experienced a postoperative MI and a postoperative stroke could be considered to contribute information about perioperative morbidity, we excluded them from all analyses to maintain one uniform cohort of patients for all analyses; in addition, they represented <1% of patients identified for study (7 of 8322), and therefore were unlikely to influence the findings in a meaningful way.
Primary end point and exposure variable
The primary study end point was survival, assessed over the first year and over the first 5 years postoperatively. Survival was determined by matching patients in the registry with the Social Security Death Index. Other associations, such as the effect of symptom status on occurrence of a postoperative complication, were not assessed in the present study. The primary exposure variable was major postoperative complication, defined as in-hospital postoperative MI, stroke, or neither; data on 30-day postoperative complications are not available within the data set. Postoperative MI was defined as any or all of (1) a troponin elevation beyond the normal upper limit, as defined by the testing laboratory, without electrocardiographic (ECG) changes, or (2) clinical symptoms (severe chest pain or radiotherapy to the left arm or jaw), or (3) ECG changes consistent with MI, as defined at each participating institution.
Although recognizing that this broadly inclusive definition is not consistent with the American Heart Association (AHA) universal definition of MI,12 we defined MI in this way to be consistent with the definition used in several previous carotid revascularization trials.2,3 In addition, a literal interpretation of the second part leaves open the possibility that patients who experienced clinical symptoms alone would be categorized as having sustained an MI. Because most practitioners would likely not consider this sufficient evidence to make that diagnosis, we have assumed that there would be very few instances of this misclassification when reporting outcomes also. We instead suspect that most often, this variable is understood to mean clinical symptoms in addition to troponin elevation, because the other levels of this nominal variable are “no MI,” or “troponin elevation only.”
It is a limitation of the data set that we are unable to assess the number of patients for whom troponin assays were sent, but were not elevated, or conversely, the number of patients who experienced ECG evidence of MI with clinical symptoms but did not have troponin assays sent; those patients in the first scenario would likely be counted as having “no MI,” whereas those in the second scenario would be counted as having sustained an MI.
Postoperative stroke included major and minor strokes, occurring in the ipsilateral or contralateral hemisphere; transient ischemic attacks were not included. The occurrence of a postoperative stroke was at the determination of the physician; the data set does not capture information such as radiographic evidence of stroke or involvement of a neurologist or other consultant.
Secondary end point
We assessed mortality within the first 30 days postoperatively, stratified according to the occurrence of postoperative stroke, postoperative MI, or neither, in order to better understand the associations of these with early death. We did not exclude these patients from our overall analyses of survival because we felt they still contributed valuable information regarding the risk of death after a particular exposure.
Covariates examined
Patient information was collected for >100 clinical and demographic variables (available at www.vsgne.org). Demographic information included age at the time of the procedure, sex, and race. Comorbidities examined included coronary artery disease (CAD, categorized as history of MI without current symptoms or stable angina, or unstable angina or MI within the past 6 months), chronic obstructive pulmonary disease (dependent on medication or home oxygen), congestive heart failure (CHF, by history), diabetes mellitus (insulin-dependent or controlled by oral medication or diet, or both), hypertension (history of hypertension or blood pressure ≥140/90 mm Hg on the preoperative evaluation), and history of tobacco use. Renal disease was categorized in three strata: normal (serum creatinine ≤1.8 mg/dL), renal insufficiency (serum creatinine >1.8 mg/dL), and dialysis-dependence. Results of preoperative stress testing were defined as not performed, negative for ischemia, or positive for ischemia, MI, or both. History of previous coronary revascularization included CABG and percutaneous coronary intervention. Contralateral internal carotid artery stenosis was classified as <50%, 50% to 69%, 70% to 79%, ≥80%, occluded, or unknown.
Preoperative medication use was recorded, including β-blockers, antiplatelet agents (aspirin, clopidogrel, or both), and statins. Anesthetic technique was categorized as general or other, which included local or regional anesthetic. Other operative information included the use of heparin and the use of protamine for reversal. Patients were classified as having symptomatic carotid artery stenosis if there was any history of ipsilateral ocular symptoms or cortical transient ischemic attack or stroke, including major and minor strokes. Hospital site was also recorded.
Statistical analysis
Descriptive statistics were used to assess incidence of postoperative MI and stroke. Univariate analyses were conducted with χ2 analysis for nominal variables; this included postoperative complication, demographics, comorbid conditions, contralateral ICA stenosis, preoperative medications, and operative factors including hospital site. Survival analyses were performed according to the Kaplan-Meier method, with intergroup comparisons made using log-rank testing. Analyses were performed on the entire cohort, with additional subgroup analyses based on procedure type. However, we intentionally limited our use of subgroup analyses because this study was neither designed nor properly powered to assess differences in revascularization strategies. Nolan et al13 recently conducted a study using VSGNE data that was designed specifically to compare CAS with CEA.
Cox proportional hazards modeling with backward stepwise selection was used to determine the magnitude of association of postoperative MI and stroke with the risk of death during the first year and 5 years postoperatively. Covariates were chosen based on a univariate screen with entry for those with P < .2. Significance was accepted at the P < .05 level. All analyses were conducted using SAS 9.2 software (SAS Institute, Cary, NC).
RESULTS
Cohort characteristics
Between January 2003 and December 2011, 8991 patients underwent carotid revascularization procedures recorded in the VSGNE database. We excluded 464 patients because data were missing for the exposure variable, postoperative stroke, or postoperative MI. Also excluded were 205 patients who underwent combined CEA and CABG and a further seven patients who experienced postoperative MI and stroke, leaving 8315 patients for analysis (Table I). CEA was performed in 7834 (94%) and CAS in 481 (6%). Most patients were men (5000 [61%]), aged between 60 and 79 years (5781 [70%]), with a history of tobacco use (6628 [80%]), CAD (2705 [32.6%], and hypertension (7288 [87.7%]). Procedures were performed for symptomatic lesions in 2933 (35.3%). High-grade stenoses (>70%) or occlusions of the contralateral ICA were present in 1924 patients (23.2%). General anesthesia was used for 6903 procedures (83%).
Table I.
Demographics for patients in the Vascular Study Group of New England cohort 2003–2011, stratified according to postoperative myocardial infarction (MI), stroke, or neither
| Covariate | Total (n = 8315), No. (%) | No MI or stroke (n = 8171), No. (%) | MI (n = 81), No. (%) | Stroke (n = 63), No. (%) | P |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | .05 | ||||
| <60 | 1260 (15.2) | 1242 (15.2) | 7 (8.6) | 11 (17.5) | |
| 60–69 | 2693 (32.4) | 2647 (32.4) | 34 (42) | 12 (19.1) | |
| 70–79 | 3088 (37.1) | 3035 (37.1) | 29 (35.8) | 24 (38.1) | |
| >80 | 1274 (15.3) | 1247 (15.3) | 11 (13.6) | 16 (25.4) | |
| Male sex | 5000 (60.1) | 4924 (60.3) | 38 (46.9) | 38 (60.3) | .05 |
| White race | 8180 (98.4) | 8039 (98.4) | 80 (98.8) | 61 (96.8) | .6 |
| Preoperative factors | |||||
| Smoking status | .63 | ||||
| Never | 1676 (20.2) | 1651 (20.2) | 13 (16.1) | 12 (19.1) | |
| Former/current | 6628 (79.8) | 6509 (79.8) | 68 (84) | 51 (81) | |
| COPD | <.0001 | ||||
| None/not treated | 7179 (86.4) | 7063 (86.5) | 63 (77.8) | 53 (84) | |
| Medications | 994 (12) | 974 (12) | 10 (12.4) | 10 (15.9) | |
| Home oxygen | 141 (1.7) | 133 (1.6) | 8 (9.9) | 0 (0) | |
| CAD | .02 | ||||
| None | 5607 (67.5) | 5520 (67.6) | 42 (51.9) | 45 (71.4) | |
| History of MI/stable angina | 2540 (30.6) | 2488 (30.5) | 35 (43.2) | 17 (27) | |
| Unstable angina; MI ≤6 months | 165 (2) | 160 (2) | 4 (5) | 1 (1.6) | |
| Congestive heart failure | 700 (8.4) | 677 (8.3) | 17 (21) | 6 (9.5) | .0002 |
| Hypertension | 7288 (87.7) | 7154 (87.6) | 74 (91.4) | 60 (95.2) | .11 |
| Diabetes mellitus | 2601 (31.3) | 2540 (31.1) | 39 (48.2) | 22 (34.9) | .004 |
| Renal function | .05 | ||||
| Creatinine ≤1.8 mg/dL | 7596 (94.1) | 7469 (94.2) | 69 (87.3) | 58 (95.1) | |
| Creatinine >1.8 mg/dL | 429 (5.3) | 416 (5.3) | 10 (12.7) | 3 (4.9) | |
| Dialysis-dependent | 45 (0.6) | 45 (0.6) | 0 (0) | 0 (0) | |
| Contralateral ICA stenosis | .41 | ||||
| <50% | 4350 (52.4) | 4281 (52.4) | 38 (46.9) | 31 (49.2) | |
| 50%–69% | 1752 (21.1) | 1723 (21.1) | 20 (24.7) | 9 (14.3) | |
| 70%–80% | 832 (10) | 819 (10) | 8 (9.9) | 5 (7.9) | |
| >80% | 521 (6.3) | 508 (6.2) | 7 (8.6) | 6 (9.5) | |
| Occluded | 571 (6.9) | 558 (6.8) | 6 (7.4) | 7 (11.1) | |
| Unknown | 281 (3.4) | 274 (3.4) | 2 (2.5) | 5 (7.9) | |
| Preoperative medications | |||||
| Antiplatelet agents | 7498 (90.2) | 7368 (90.2) | 75 (92.6) | 55 (87.3) | .57 |
| β-blockers | 6492 (78.1) | 6373 (78) | 69 (85.2) | 50 (79.4) | .29 |
| Statins | 6359 (76.5) | 6244 (76.4) | 67 (82.7) | 48 (76.2) | .61 |
| Operative factors | |||||
| General anesthesia | 6903 (83) | 6782 (83) | 71 (87.7) | 50 (79.4) | .4 |
| Heparin | 8250 (99.2) | 8108 (99.3) | 79 (97.5) | 63 (100) | .16 |
| Protamine | 4173 (50.2) | 4093 (50.1) | 45 (55.6) | 35 (55.6) | .43 |
| Procedure type | .002 | ||||
| CAS | 481 (5.8) | 468 (5.7) | 3 (3.7) | 10 (15.9) | |
| CEA | 7834 (94.2) | 7703 (94.3) | 78 (96.3) | 53 (84.1) | |
| Symptomatic | 2933 (35.3) | 2865 (35.1) | 33 (40.7) | 35 (55.5) | .002 |
CAD, Coronary artery disease; CAS, carotid artery stenting; CEA, carotid endarterectomy; COPD, chronic obstructive pulmonary disease; ICA, internal carotid artery.
Postoperative MI, stroke, and long-term survival
More patients experienced postoperative MI (81 [0.97%]) than those who experienced postoperative stroke (63 [0.76%]; Table II). On univariate analysis, the incidence of postoperative MI or stroke was 133 (1.7%) in those undergoing CEA, which was significantly lower than the 13 incidence (2.7%) in CAS patients (P = .002). On analysis of each end point separately, a greater number of MIs occurred after CEA (78 [1.0%]) than after CAS (3 [0.62%]), whereas a greater number of strokes occurred after CAS (10 [2.1%]) than after CEA (53 [0.68%]).
Table II.
Event rates stratified by procedure type (P = .002)
| Procedure type | No MI or stroke, No. (%) | Postoperative
|
|
|---|---|---|---|
| MI, No. (%) | Stroke, No. (%) | ||
| Total cohort | 8171 (98.3) | 81 (.97) | 63 (.76) |
| CEA | 7703 (98.3) | 78 (1.0) | 53 (.68) |
| CAS | 468 (97.3) | 3 (.62) | 10 (2.1) |
CAS, Carotid artery stenting; CEA, carotid endarterectomy; MI, myocardial infarction.
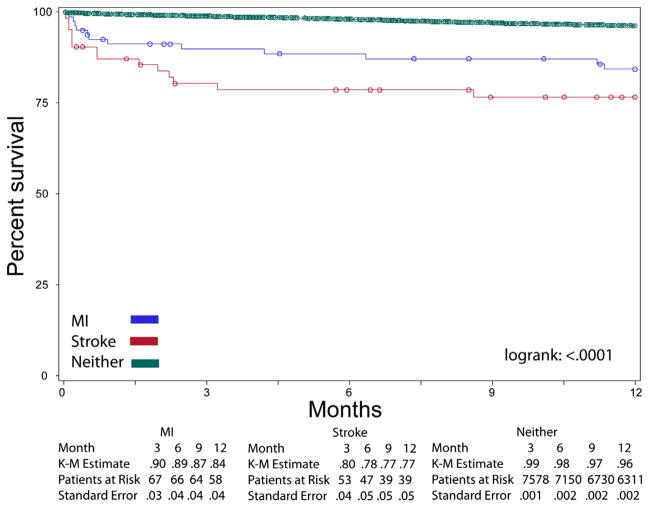
During the first year, Kaplan-Meier survival analysis revealed a significant difference when the cohort was stratified according to occurrence of postoperative MI, stroke, or neither (Fig 1). Among those without MI or stroke, the 1-year survival was 96% (standard error [SE], 0.22%), whereas survival was 84% (SE, 4.1%) among those with a postoperative MI and 77% (SE, 5.5%) among those with a postoperative stroke.
Fig 1.
Kaplan-Meier (K-M) curves of survival over the first year postoperatively, stratified according to postoperative myocardial infarction (MI), stroke, or neither.
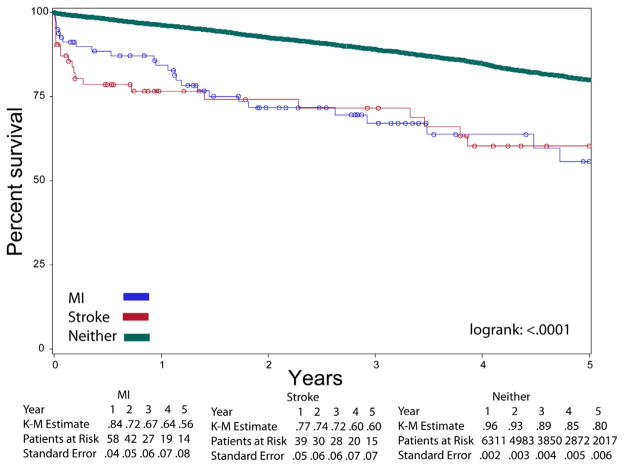
During the first 5 years, Kaplan-Meier survival analysis revealed a significant difference when the cohort was stratified according to occurrence of postoperative MI, stroke, or neither (Fig 2). However, a convergence of the Kaplan-Meier curves occurred at the 5-year point, with similar survival between the postoperative MI group and the postoperative stroke group. The 5-year survival was 80% (SE, 0.62%) among those without MI or stroke, 56% (SE, 7.8%) among those with a postoperative MI, and 60% (SE 7.4) for those with postoperative stroke.
Fig 2.
Kaplan-Meier (K-M) curves of survival over the first 5 years postoperatively, stratified according to postoperative myocardial infarction (MI), stroke, or neither.
Thirty-day mortality
Life-table analysis revealed that the 30-day mortality among patients without MI or stroke was 0.5% (SE, 0.07%). Thirty-day mortality was 8.7% (SE, 3.2%) among those with postoperative MI and 12.8% (SE, 4.2) among those with postoperative stroke.
Independent predictors of 1-year survival
On multivariable analysis, postoperative MI and stroke were both predictors of worse survival during the first 1 year. Survival during the first year (hazard ratio [HR], 3.6; 95% confidence interval [CI], 2.0–6.8; P < .0001) for those with postoperative MI was significantly less than for those who did not experience a postoperative complication (Table III). Similarly, survival during the first year was significantly (HR, 6.6; 95% CI, 3.7–11.9, P < .0001) less for those with postoperative stroke than for those who did not experience a postoperative complication (Table III).
Table III.
Cox proportional hazards model of survival over the first year postoperatively
| Covariate | HR (95% CI) | P |
|---|---|---|
| Postoperative complication | ||
| No stroke or MI | Referent | |
| MI | 3.6 (2.0–6.8) | <.0001 |
| Stroke | 6.6 (3.7–11.9) | <.0001 |
| Age, years | ||
| <60 | Referent | |
| 60–69 | 0.81 (0.51–1.3) | .4 |
| 70–79 | 1.6 (1.1–2.5) | .02 |
| >80 | 3.2 (2.1–4.9) | <.0001 |
| COPD | ||
| None/not treated | Referent | |
| Medications | 1.1 (0.78–1.6) | .57 |
| Home oxygen | 3.8 (2.4–6.0) | <.0001 |
| CAD | ||
| None | Referent | |
| History of MI or stable angina | 1.1 (0.79–1.4) | .72 |
| Unstable angina or MI ≤6 months | 1.4 (0.69–3.0) | .33 |
| Previous coronary revascularization | 1.1 (0.80–1.4) | .67 |
| Congestive heart failure | 1.7 (1.3–2.4) | .001 |
| Diabetes mellitus | 1.4 (1.1–1.7) | .01 |
| Renal function | ||
| Creatinine ≤1.8 mg/dL | Referent | |
| Creatinine >1.8 mg/dL | 1.7 (1.2–2.5) | .004 |
| Dialysis-dependent | 6.1 (3.4–11) | <.0001 |
| Contralateral ICA stenosis | ||
| <50% | Referent | |
| 50%–69% | 1.0 (0.74–1.4) | .89 |
| 70%–80% | 1.5 (1.0–2.1) | .04 |
| >80% | 2.2 (1.5–3.2) | <.0001 |
| Occluded | 1.3 (0.82–2.1) | .26 |
| Unknown | 1.6 (0.91–2.9) | .1 |
| Preoperative β-blocker use | 0.89 (0.67–1.2) | .42 |
| Preoperative statin use | 0.81 (0.62–1.0) | .1 |
| General anesthesia | 0.75 (0.56–1.0) | .05 |
CAD, Coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; ICA, internal carotid artery; MI, myocardial infarction.
Independent predictors of 5-year survival
Multivariable analysis of survival during the first 5 years postoperatively showed that postoperative MI and stroke were significant predictors of worse survival. Survival during the first 5 years (HR, 2.8; 95% CI, 1.8–4.3; P < .0001) was significantly less for those with postoperative MI than for those who did not experience a postoperative complication (Table IV). Postoperative stroke was also associated with a reduced survival during the first 5 years (HR, 2.7; 95% CI, 1.7–4.3; P < .0001) compared with those who did not experience a postoperative complication.
Table IV.
Cox proportional hazards model of survival over the first 5 years postoperatively
| Covariate | HR (95% CI) | P |
|---|---|---|
| Postoperative complication | ||
| No stroke or MI | Referent | |
| MI | 2.8 (1.8–4.3) | <.0001 |
| Stroke | 2.7 (1.7–4.3) | <.0001 |
| Age, years | ||
| <60 | Referent | |
| 60–69 | 1.1 (.86–1.4) | .44 |
| 70–79 | 2.1 (1.6–2.6) | <.0001 |
| >80 | 4.5 (3.5–5.8) | <.0001 |
| Smoking status, never | 0.65 (.54–.77) | <.0001 |
| COPD | ||
| None/not treated | Referent | |
| Medications | 1.5 (1.3–1.8) | <.0001 |
| Home oxygen | 3.3 (2.5–4.5) | <.0001 |
| CAD | ||
| None | Referent | |
| History of MI or stable angina | 1.2 (.99–1.4) | .06 |
| Unstable angina or MI ≤6 months | .97 (.58–1.6) | .89 |
| Previous coronary revascularization | 0.99 (.85–1.2) | .87 |
| Stress testing | ||
| Not performed | 1.0 (.88–1.2) | .72 |
| Normal | Referent | |
| Ischemia/MI/both | 1.2 (.96–1.5) | .12 |
| Congestive heart failure | 1.7 (1.4–2.1) | <.0001 |
| Hypertension | .89 (.73–1.1) | .25 |
| Diabetes mellitus | 1.4 (1.2–1.6) | <.0001 |
| Renal function | ||
| Creatinine ≤1.8 mg/dL | Referent | |
| Creatinine >1.8 mg/dL | 1.6 (1.3–1.9) | <.0001 |
| Dialysis-dependent | 5.3 (3.5–7.9) | <.0001 |
| Contralateral ICA stenosis | ||
| <50% | Referent | |
| 50%–69% | 1.1 (.96–1.3) | .15 |
| 70%–80% | 1.3 (1.1–1.6) | .008 |
| >80% | 1.6 (1.3–2.1) | <.0001 |
| Occluded | 1.6 (1.3–2.1) | <.0001 |
| Unknown | 1.4 (1.0–2.0) | .04 |
| Preoperative antiplatelet use | 1.0 (.83–1.2) | .95 |
| Preoperative statin use | .80 (.70–.92) | .002 |
| General anesthesia | .77 (.66–.91) | .002 |
CAD, Coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; ICA, internal carotid artery; MI, myocardial infarction.
DISCUSSION
This study demonstrates that patients experiencing an MI or a stroke after carotid revascularization have significant reductions in survival over the first 1 and 5 years compared with patients experiencing neither complication. However, postoperative MI and stroke have different implications. During the first year, postoperative stroke conferred nearly twice the hazard of reduced survival as MI (HR, 6.6 vs 3.6). By 5 years, the magnitude of association of these two postoperative complications was similar, with both conferring a nearly threefold reduction in survival compared with patients experiencing neither complication (stroke: HR, 2.7; MI: HR, 2.8). Other predictors of reduced long-term survival included advanced age, chronic obstructive pulmonary disease, CHF, diabetes mellitus, impaired renal function, and higher degrees of contralateral ICA stenosis.
Despite postoperative MI demonstrating a significant association with decreased survival, history of CAD was not significant in the 1-year or 5-year models. Although this seems somewhat counterintuitive, it may be that CAD, as manifested by ischemic symptoms, is not associated with reduced survival, but rather that CAD associated with CHF is, because CHF is a significant independent predictor in both models. This may reflect the degree to which cardiac function has been affected as the mediator of reduced survival.
Several studies have characterized outcomes after CEA and CAS, although most have used composite end points. This has partly contributed to the controversy surrounding determination of the optimal revascularization strategy. Wang et al14 evaluated predictors of 1-year outcomes, focusing primarily on the effect of each procedure type, CEA vs CAS, on survival. However, they did not specifically evaluate the effect on survival of a postoperative MI or stroke after carotid revascularization. The SAPPHIRE trial assessed outcomes among patients at high risk for CEA due to medical comorbidities and found noninferiority of CAS for the composite end point of perioperative MI, stroke, and death.2 The SPACE trial evaluated outcomes for CAS and CEA among patients with symptomatic carotid stenosis7 and concluded that CAS was inferior to CEA for the composite end point of stroke and death. The difference in conclusions may have related to a difference in end points.
Two of the largest trials to date included subgroup analyses of single end points. The International Carotid Stenting Study evaluated outcomes of CEA and CAS for symptomatic carotid stenosis and found a higher risk of stroke at 30 days associated with CAS.5 The CREST trial included symptomatic and asymptomatic carotid stenosis, and no significant difference was found between the two revascularization strategies for a composite end point of stroke, MI, and death.3 However, subgroup analysis revealed higher risk of stroke associated with CAS and higher risk of MI with CEA.
The technique of meta-analysis has also been used in an attempt to clarify these conflicting results and multiple study end points.15 Using a pooled analytic approach for their meta-analysis, Wang et al found that in the perioperative period, similar to the findings of the CREST trial, CEA was associated with higher rates of MI, whereas CAS was associated with higher rates of stroke. This provided good statistical evidence that the two revascularization strategies have different likelihoods of these two complications being associated with them. Therefore, the next step in interpretation of these studies requires an analysis of the relative effect of these complications on other outcomes such as long-term survival.
Our primary analysis was a characterization of the effect of postoperative MI and stroke on 1-year and 5-year survival after carotid revascularization. The utility of evaluating these two exposures separately was further supported by a secondary substudy in the CREST trial that evaluated health-related quality of life (HRQOL).16 They reported the effect of revascularization type on HRQOL and found few differences between CAS and CEA by 1 year. However, subgroup analyses showed the occurrence of a postoperative stroke was associated with worse HRQOL for seven of eight health-status domains. In contrast, those who experienced a postoperative MI reported only a worse general health perception at 1 year, but no other significant differences in HRQOL. The authors concluded that these complications had different implications for HRQOL, whereas procedure type made little difference by 1 year postoperatively.
The aforementioned studies evaluated patient outcomes according to revascularization strategy, but the effect of individual adverse outcomes (MI or stroke) was not addressed or was assessed only as a post hoc subgroup analysis and was therefore subject to significant inherent limitations. In contrast, our primary analysis focused specifically on the effect of these serious postoperative events. Our findings build on the findings from the CREST HRQOL study that suggested that postoperative stroke and MI have different implications for patients after carotid revascularization. The occurrence of a postoperative MI, or a postoperative stroke, portended a worse early survival prognosis, with stroke affecting 1-year survival far more than MI.
It is also important to note that the association we have identified does not demonstrate cause and effect. For instance, a postoperative MI may occur more commonly in a patient with more severe underlying CAD that would lead to reduced survival. Conversely, a postoperative MI may lead to worsening cardiac function, with a subsequent decline in overall health that leads to reduced survival compared with a patient who did not experience a postoperative MI. Our analyses do not allow for interpretation of the direction of the association, only that there is an association of postoperative stroke and postoperative MI with an increased hazard of death at 1 and 5 years postoperatively compared with having neither of these postoperative complications.
The event rate in this study is lower than that reported in many other studies. For example, the CREST investigators reported an incidence of 6.4% for stroke and 3.4% for MI in the periprocedural period,3 which is substantially higher than the rates observed in the VSGNE cohort. This may represent a limitation of self-reporting in VSGNE. In an attempt to reduce this type of potential bias, VSGNE has validated the occurrence of postoperative stroke using International Classification of Diseases-9th edition codes for this complication on hospital administrative claims for CEA.
A recent publication from Nolan et al13 detailed this method. They found that that 8% of CEA performed according to administrative claims had not been entered into the VSGNE. On review of administrative claims that included a code for postoperative iatrogenic stroke, 100% of these had been entered properly into the VSGNE; that is, none of the cases that had been identified as missing from VSGNE contained a code for postoperative stroke. Furthermore, the VSGNE does not mandate an independent neurologic examination before and after the procedure, as is often the case in carotid revascularization trials. As with all self-reported databases, the reliance on the physician who performed the revascularization to report the occurrence of a postoperative stroke does leave open the possibility of an under-reporting bias. Although it would be preferable to mandate independent postoperative neurologic evaluations for all patients after carotid revascularization, this is not feasible within the VSGNE.
As mentioned previously, the definition used for MI does not conform to the AHA universal definition of MI12; instead, it includes isolated troponin elevations. This decision was made deliberately to be consistent with the definition used in several previous carotid revascularization trials. Our definition varies from the AHA universal definition by not requiring other clinical markers of ischemia such as chest pain or ECG changes. Given the way the variable is defined in the data set, this means that patients who had a troponin elevation without necessarily demonstrating ECG changes or chest pain would be included as having sustained this postoperative complication. Because of the limitations of the data set, we are not able to determine the number of patients for whom troponin assays were sent, but not elevated. The likely effect of using this more inclusive MI definition would be an underestimation of death secondary to inclusion of patients who experienced a less severe cardiac event. Accordingly, the association of MI with reduced survival might have been more pronounced if we had adhered strictly to this AHA definition. A corollary to this is the recognition that there may be an association of isolated postoperative troponin elevations that fall short of the criteria for MI with decreased survival.
It is important to state explicitly that the purpose of this investigation was not to compare the effectiveness of CEA and CAS. In contrast, this study was designed to specifically assess the association of a postoperative MI or a postoperative stroke with reduced long-term survival, compared with neither complication, after carotid revascularization. As a result, these data do not allow us to make conclusions regarding the optimal carotid revascularization strategy. Other VSGNE investigators have addressed the comparative effectiveness question between CEA and CAS.13
CONCLUSIONS
Postoperative stroke and postoperative MI affect patients differently, with stroke being significantly associated with more early death than MI. This analysis informs the interpretation of the various studies that have used a composite end point of stroke, MI, and death. It also highlights the need for future studies of the comparative effectiveness of CEA and CAS to evaluate postoperative stroke and postoperative MI separately because they have different implications for HRQOL and survival.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: JS, AS
Analysis and interpretation: JS, PG, DB, BN, NH, JC, LM, AS
Data collection: AS
Writing the article: JS, AS
Critical revision of the article: JS, PG, DB, BN, JC, LM, AS
Final approval of the article: JS, PG, DB, BN, NH, JC, LM, AS
Statistical analysis: JS, NH, AS
Obtained funding: Not applicable
Overall responsibility: AS
Author conflict of interest: none.
Presented at the Thirty-ninth Annual Meeting of the New England Society for Vascular Surgery, Boston, Mass, September 21–23, 2012.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–71. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 2.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotidartery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 3.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotidartery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902. doi: 10.1016/S1474-4422(08)70196-0. [DOI] [PubMed] [Google Scholar]
- 5.Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–97. doi: 10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jim J, Rubin BG, Ricotta JJ, 2nd, Kenwood CT, Siami FS, Sicard GA. Society for Vascular Surgery (SVS) Vascular Registry evaluation of comparative effectiveness of carotid revascularization procedures stratified by Medicare age. J Vasc Surg. 2012;55:1313–20. doi: 10.1016/j.jvs.2011.11.128. discussion: 1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–47. [Google Scholar]
- 8.Dumont TM, Rughani AI. National trends in carotid artery revascularization surgery. J Neurosurg. 2012;116:1251–7. doi: 10.3171/2012.3.JNS111320. [DOI] [PubMed] [Google Scholar]
- 9.Goodney PP, Travis LL, Malenka D, Bronner KK, Lucas FL, Cronenwett JL, et al. Regional variation in carotid artery stenting and endarterectomy in the Medicare population. Circ Cardiovasc Qual Outcomes. 2010;3:15–24. doi: 10.1161/CIRCOUTCOMES.109.864736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel MR, Greiner MA, DiMartino LD, Schulman KA, Duncan PW, Matchar DB, et al. Geographic variation in carotid revascularization among Medicare beneficiaries, 2003–2006. Arch Intern Med. 2010;170:1218–25. doi: 10.1001/archinternmed.2010.194. [DOI] [PubMed] [Google Scholar]
- 11.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion: 1101–2. [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 13.Nolan BW, De Martino RR, Goodney PP, Schanzer A, Stone DH, Butzel D, et al. Comparison of carotid endarterectomy and stenting in real world practice using a regional quality improvement registry. J Vasc Surg. 2012;56:990–6. doi: 10.1016/j.jvs.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang FW, Esterbrooks D, Kuo YF, Mooss A, Mohiuddin SM, Uretsky BF. Outcomes after carotid artery stenting and endarterectomy in the Medicare population. Stroke. 2011;42:2019–25. doi: 10.1161/STROKEAHA.110.608992. [DOI] [PubMed] [Google Scholar]
- 15.Economopoulos KP, Sergentanis TN, Tsivgoulis G, Mariolis AD, Stefanadis C. Carotid artery stenting versus carotid endarterectomy: a comprehensive meta-analysis of short-term and long-term outcomes. Stroke. 2011;42:687–92. doi: 10.1161/STROKEAHA.110.606079. [DOI] [PubMed] [Google Scholar]
- 16.Cohen DJ, Stolker JM, Wang K, Magnuson EA, Clark WM, Demaerschalk BM, et al. Health-related quality of life after carotid stenting versus carotid endarterectomy: results from CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial) J Am Coll Cardiol. 2011;58:1557–65. doi: 10.1016/j.jacc.2011.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]