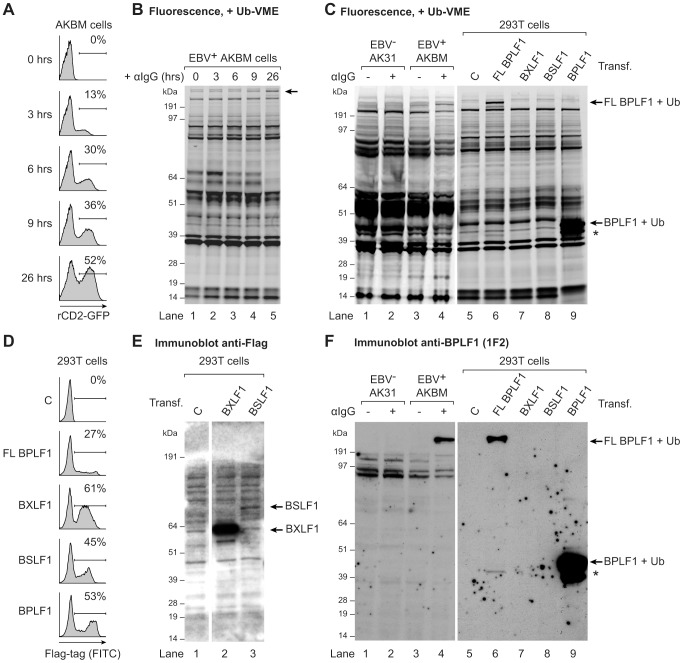
Figure 1. EBV BPLF1 is a DUB active during productive infection.
EBV+ AKBM BL cells were treated with anti-human IgG (αIgG) to induce viral replication. (a) At the indicated times post induction, percentages of productively EBV-infected AKBM cells were determined by flow cytometric analysis of induced ratCD2-GFP reporter expression. (b) In post-nuclear AKBM cell lysates, active DUBs were labeled with a fluorescent Ub-VME probe, resolved by SDS-PAGE, and visualized by in-gel fluorescence imaging. The arrow indicates a band appearing in AKBM cells after 9 hours of lytic cycle induction (lanes 4 and 5). (c) DUB profiles of EBV− AK31-rCD2-GFP cells (AK31, lanes 1 and 2), EBV+ AKBM cells (lanes 3 and 4), and transfected 293T cells (lanes 5–9). In parallel with the EBV+ AKBM cells, EBV− control AK31 cells were treated with αIgG for 24 hours; this resulted in productive infection in 56% of AKBM cells; AK31 cells included a population of 45% that expressed ratCD2-GFP from a constitutive promoter irrespective of αIgG treatment (data not shown). For comparison, 293T cells were transfected with constructs encoding three (putative) EBV DUBs: BPFL1, BXLF1, BSLF1, or the 325 aa N-terminal part of BPLF1. The asterisk marks a smaller fragment observed upon transfection of 293T cells with full-length or the N-terminal part of BPLF1 (lanes 6 and 9). Left and right panels are parts of one gel displayed at different exposures. (d) Sixteen hours post-transfection, percentages of 293T cells expressing BPLF1, BXLF1, BSLF1 or the N-terminal fragment of BPLF1 were determined by intracellular FACS staining for the Flag-tag. (e) Immunoblot of part of the gel in c probed with an anti-Flag Ab to detect transfected BXLF1 and BSLF1 (sequential staining following 1F2, see in f). (f) Immunoblot of the gel in c probed with the BPLF1-specific mouse monoclonal Ab 1F2. Both panels are part of one gel presented at different exposures.