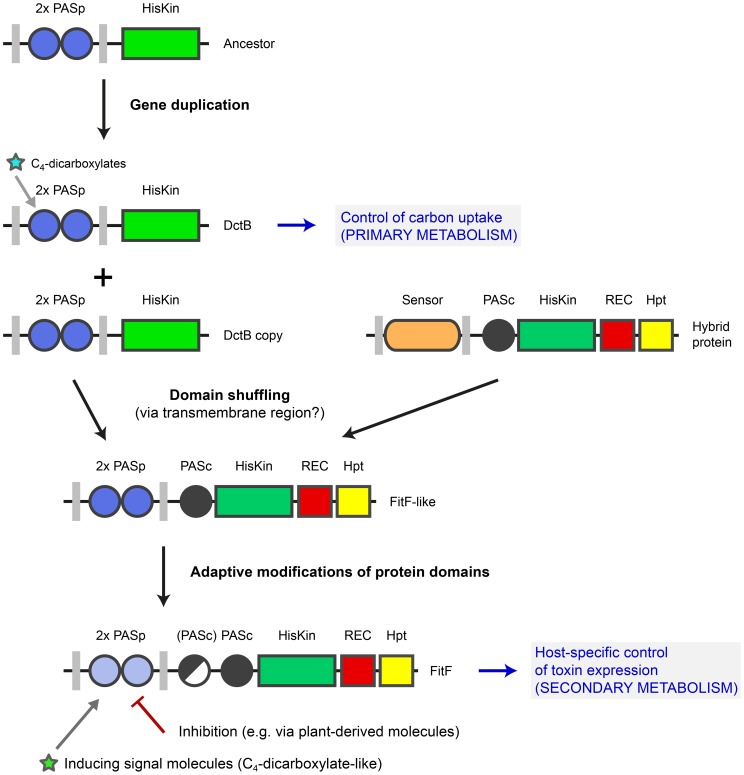
Figure 7. Model for evolution of FitF via a domain shuffling event involving a DctB ancestor.
The ancestor of the gene coding for the sensor kinase DctB was duplicated several times in various proteobacterial species. One dctB gene copy underwent a fusion with a gene encoding a histidine kinase-response regulator hybrid protein, possibly by homologous recombination via a conserved region coding for the second transmembrane region of the sensor proteins. This domain shuffling event resulted in the expression of a hybrid histidine kinase with a dual PASp domain architecture in the periplasmic portion. Selective pressure then led to adaptive modifications in the protein sequence and domain topology (i.e. insertion of a second PASc domain in P. chlororaphis). Domain shuffling and subsequent modifications during the evolution of FitF significantly contributed to the ability of P. protegens CHA0 to produce its insecticidal toxin in a host-specific manner and as a result to the evolution of insect pathogenicity in this biocontrol bacterium. Inhibition of FitF by plant-derived molecules may be a mechanism helping the bacterium to distinguish between the plant and insect host. The evolution of FitF may have taken place in bacterial species other than P. protegens, implying horizontal gene transfer.