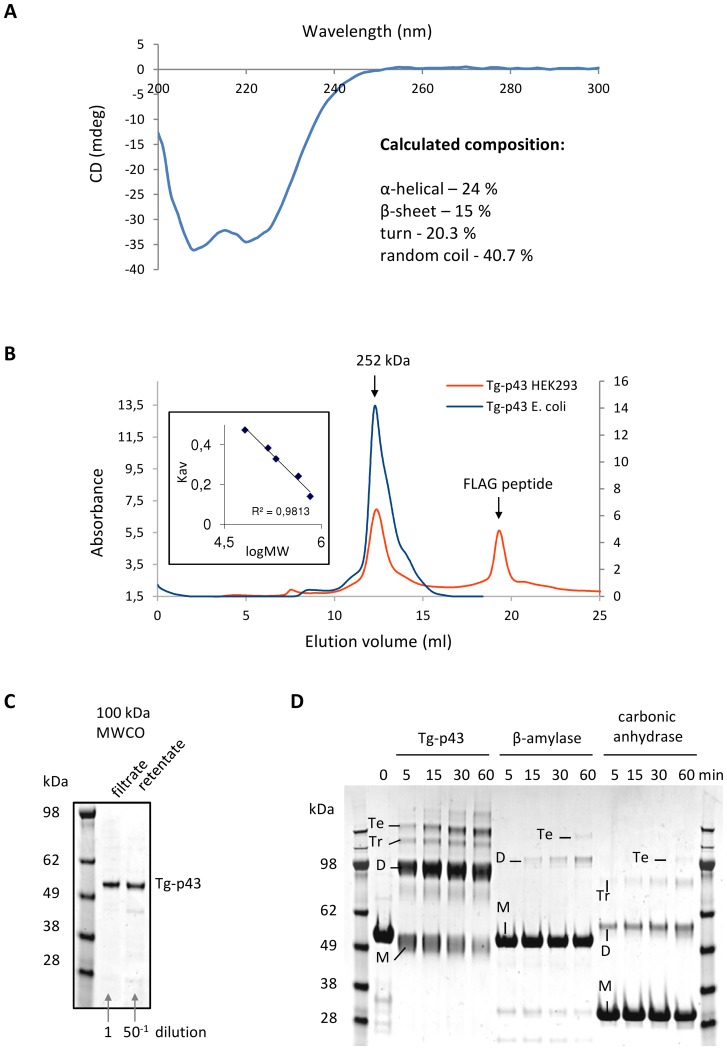
Figure 2. Characterization of the folding and oligomeric state of recombinant Tg-p43.
(A) Circular dichroism spectrum of recombinant Tg-p43 expressed and purified from E. coli. The percentages of the calculated secondary structures are given in the inset. (B) SEC profile of pure recombinant E. coli (blue) and HEK293 (orange)-derived Tg-p43 protein. The MW of the eluted proteins, calculated by calibration with HMW standards (inset left), is shown above the trace. (C) Coomassie blue-stained SDS-PAGE gel image of E.coli-derived Tg-p43 retained and passed by a 100 kDa MWCO ultrafiltration device. (D) Coomassie blue-stained SDS-PAGE gel comparing the susceptibility to cross-linking (glutaraldehyde) of E.coli-derived Tg-p43 vs. beta-amylase (tetrameric oligomer) and carbonic anhydrase (monomeric) proteins. Based on size considerations, bands have been labeled as either monomers (M), dimers (D), trimers (Tr), or tetramers (Te).