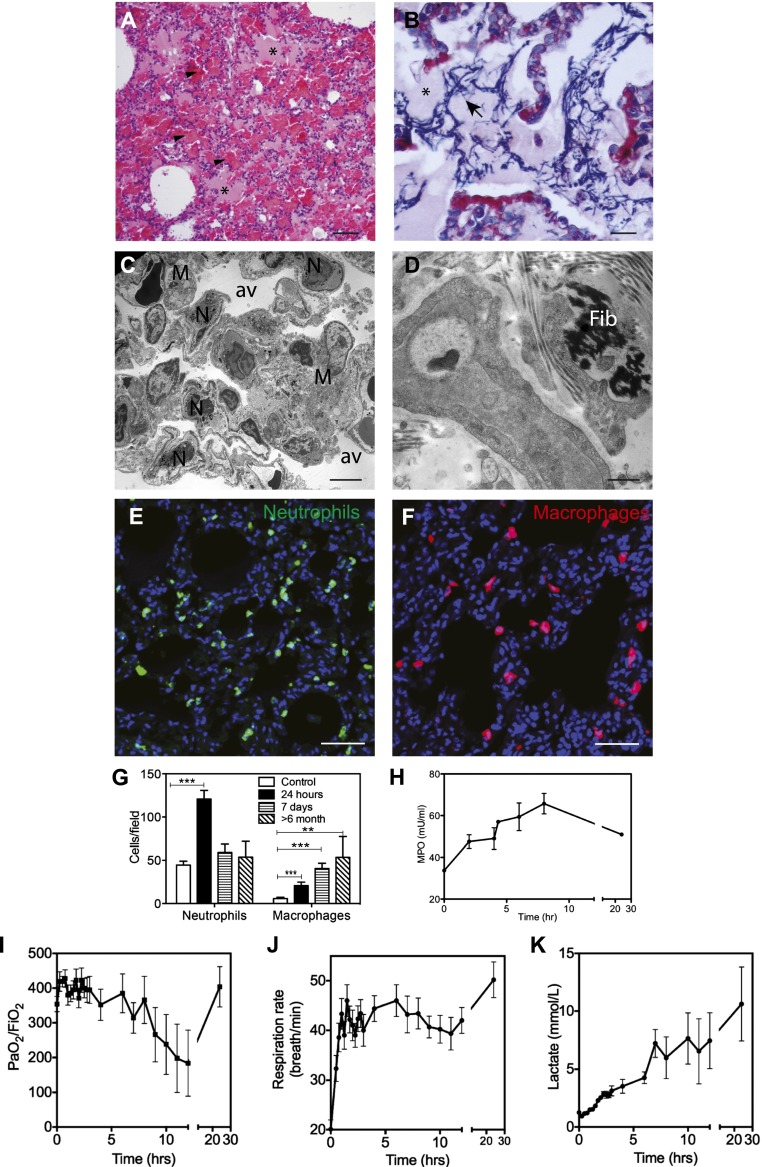
Figure 1.
Histological and physiological changes of the lung during the acute exudative phase of acute respiratory distress syndrome (ARDS) in the baboon model of Escherichia coli sepsis. (A and B) Hematoxylin and eosin (A) and phosphotungstic acid\x{2013}hematoxylin (PTAH) (B) staining show the presence of protein-rich edema fluid filling the alveoli (A and B, asterisks), spotty intra-alveolar hemorrhage (A, arrowheads), and intra-alveolar fibrin deposition (B, blue staining, arrows). (C and D) Electron micrographs, showing (C) neutrophil (N) and macrophage (M) accumulation, and (D) the presence of interstitial fibrin (Fib), suggest the activation of coagulation. (E and F) Immunostaining of elastase-stained neutrophils (E) and CD68-positive macrophages (F) in the lung of healthy control and septic baboons after 24 hours after challenge. Nuclei are shown in blue. (G) Quantitation of neutrophils and macrophages in the lung during the time course of the study. Histogram data are shown as means ± SEM (n = 10 microscopic fields collected from at least three different animals per condition per time point; one-way ANOVA with Dunnett’s multicomparison test, **P < 0.01, ***P < 0.001). (H) Time course changes of plasma myeloperoxidase suggest gradual neutrophil activation and degranulation. (I–K) Biochemical and functional tests demonstrate that E. coli–challenged baboons showed clinical signs of ARDS: abrupt decrease of blood oxygenation (I), increased respiratory rate (tachypnea) (J), and gradual increase in plasma lactate (K). Data are shown as means ± SEM (n = 4). Scale bars, 200 μm (A and B), 10 μm (C and D), 100 μm (E and F).