Abstract
Non-alcoholic fatty liver disease (NAFLD) describes a range of conditions caused by fat deposition within liver cells. Liver fat content reflects the equilibrium between several metabolic pathways involved in triglyceride synthesis and disposal, such as lipolysis in adipose tissue and de novo lipogenesis, triglyceride esterification, fatty acid oxidation and very-low-density lipoprotein synthesis/secretion in hepatic tissue. In particular, it has been demonstrated that hepatic de novo lipogenesis plays a significant role in NAFLD pathogenesis. It is widely known that the fatty acid composition of the diet influences hepatic lipogenesis along with other metabolic pathways. Therefore, dietary fat may not only be involved in the pathogenesis of hepatic steatosis, but may also prevent and/or reverse hepatic fat accumulation. In this review, major data from the literature about the role of some dietary fats as a potential cause of hepatic fat accumulation or as a potential treatment for NAFLD are described. Moreover, biochemical mechanisms responsible for an increase or decrease in hepatic lipid content are critically analyzed. It is noteworthy that both quantitative and qualitative aspects of dietary fat influence triglyceride deposition in the liver. A high-fat diet or the dietary administration of conjugated linoleic acids induced hepatic steatosis. In contrast, supplementation of the diet with krill oil or pine nut oil helped in the prevention and/or in the treatment of steatotic liver. Quite interesting is the “case” of olive oil, since several studies have often provided different and⁄or conflicting results in animal models.
Keywords: Hepatic steatosis, Non-alcoholic fatty liver, Fatty acids, Lipogenesis
Core tip: Dietary fats may not only influence the pathogenesis of liver diseases, but may also prevent and/or reverse their expression. This manuscript reviews the molecular mechanisms responsible for the regulation of hepatic lipogenesis, through which some fatty acids may be beneficial or detrimental to non-alcoholic fatty liver disease (NAFLD). We believe that an understanding of the biochemical mechanisms underlying fat accumulation in the liver will lead to more targeted and effective therapeutics for hepatic steatosis. This is a particularly important topic because NAFLD is an increasingly prevalent disease which, to date, has no proven pharmacologic treatment to prevent or reverse its course.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a condition that is caused by the pathological accumulation of fat in liver. NAFLD affects 10%-35% of the current world population. In the great majority of patients, NAFLD develops in association with obesity, type 2 diabetes, insulin resistance and other metabolic abnormalities, including hypertension and dyslipidemia, collectively termed as “metabolic syndrome”[1,2].
The severity of the disease ranges from simple steatosis to acute steatohepatitis, but the pathogenesis and the molecular mechanisms controlling its progression are poorly understood. The classical pathogenesis of NAFLD is based on the “two-hit hypothesis”[3,4]. According to this hypothesis, hepatic triglyceride accumulation[5], or steatosis, represents the “first hit”, which then sensitizes the liver to injury mediated by a “second hit”, such as the secretion of proinflammatory and prothrombotic adipocytokines and the reduced production of the adipocytokine adiponectin, a potent anti-inflammatory and insulin-sensitizing agent[6-8]. In addition, mitochondrial dysfunction and oxidative stress trigger an inflammatory and fibrogenic cascade in the primed liver[3]. These events lead to steatohepatitis and fibrosis. More recently, Serviddio et al[9] underlined the importance of mitochondria in NAFLD prevention since these organelles play fundamental roles in fat metabolism and energy homeostasis, thereby counteracting the excessive accumulation of liver triglycerides.
Indeed, the main feature of NAFLD pathogenesis, both histologically and metabolically, is the accumulation of triglycerides in the liver. Although the increased mobilization of free fatty acids from adipose tissue mainly contributes to fatty liver, the specific origin of the lipids that accumulate in the liver remains unknown. Therefore, the understanding of the molecular mechanisms leading to the accumulation of lipids into the liver of NAFLD patients is of importance for the prevention and/or the reversal of this condition.
In this review, we focus our attention on the de novo lipogenesis which plays a significant role in the pathogenesis of NAFLD[10-13]. It is widely known that hepatic lipogenesis is strictly regulated by several nutritional factors[14,15], such as the fatty acid composition of the diet[16]. Such knowledge will eventually translate into the development of novel treatment strategies for NAFLD.
ROLE OF DE NOVO LIPOGENESIS IN THE DEVELOPMENT OF HEPATIC STEATOSIS
Steatosis occurs when there is an imbalance between lipid availability through fatty acid uptake and de novo lipogenesis, and lipid secretion and disposal via free fatty acid oxidation[17,18]. Figure 1 depicts the main pathways involved in fatty acid metabolism in liver and shows that a perturbed balance between triglyceride synthesis and triglyceride disposal leads to hepatic steatosis.
Figure 1.
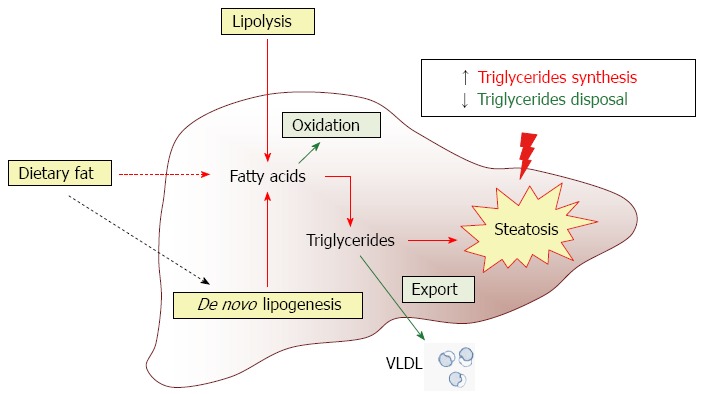
Contribution of various metabolic pathways to hepatic steatosis. Liver fat content reflects the equilibrium between several metabolic pathways involved in triglyceride synthesis (red arrows) and clearance (green arrows). Dietary fat is an important factor capable of influencing de novo lipogenesis (black dotted arrow).
As shown in Figure 1, the potential sources of fatty acids contributing to fatty liver are the non-esterified fatty acid pool from adipose tissue, dietary fatty acids and newly made fatty acids within the liver through de novo lipogenesis[13,18]. Modulation of any of the multiple mechanisms involved in lipid accumulation in the liver could provide useful targets to prevent the development of NAFLD.
To establish the relative contribution of lipid accumulation in patients with NAFLD, Donnelly et al[10] used a multiple-stable-isotope approach. These authors demonstrated that approximately 60% of liver triglyceride content was derived from free fatty acid influx from adipose tissue, 26% from de novo lipogenesis, and 15% from the diet. Other studies, carried out in animal models, provided further evidence that lipogenesis plays a key role in the development of hepatic steatosis[12]. In particular, the use of genetically engineered mice have helped to clarify that knockdown of enzymes involved in fatty acid synthesis was able to reverse NAFLD[11].
De novo fatty acid synthesis implies a complex series of reactions starting in the mitochondrial matrix and continuing in the cytosol of hepatocytes (Figure 2). The main fuel for fatty acid synthesis is acetyl-CoA derived from carbohydrate or amino acid catabolism. Since acetyl-CoA is formed in the mitochondrion, and fatty acid synthesis occurs in the cytosol, the acetyl group must be exported from the intra-mitochondrial to the extra-mitochondrial compartment of the cell before its conversion into fatty acids. Actually, in the mitochondrial matrix, acetyl-CoA is at first condensed with oxaloacetate, thereby forming the tricarboxylate citrate, an intermediate of the Krebs cycle. When this intermediate cannot be burned in the Krebs cycle because of an excess of cellular energy, it is exported from the mitochondrial matrix into the cytosol by the mitochondrial tricarboxylate carrier or citrate carrier (CIC). This carrier protein is firmly inserted into the inner mitochondrial membrane, where it catalyzes the efflux of citrate from the matrix towards the cytosol, thus playing an important role in intermediary metabolism[19]. In fact, in the cytosol the transported citrate generates acetyl-CoA, which now represents the primer for de novo fatty acid and cholesterol biosyntheses[20]. As shown in Figure 2, cytosolic fatty acid synthesis begins with the conversion of acetyl-CoA to malonyl-CoA in the reaction catalyzed by acetyl-CoA carboxylase (ACC). Next, the sequential extension of an alkanoic chain, two carbons at a time, is catalyzed by fatty acid synthase (FAS) which eventually leads to palmitic acid (16:0), the main product of de novo fatty acid synthesis.
Figure 2.
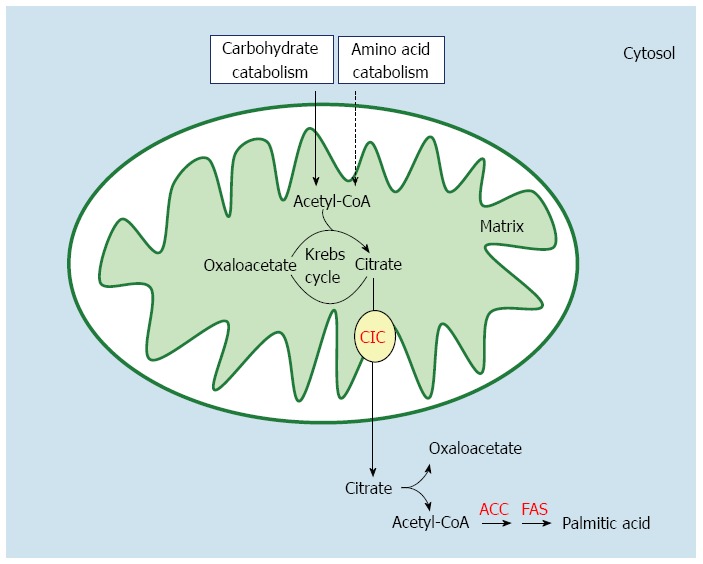
Pathway of de novo fatty acid synthesis in the liver. CIC: Citrate carrier; ACC: Acetyl-CoA carboxylase; FAS: Fatty acid synthase.
The high rate of lipogenesis, observed in hepatic steatosis, seems to be associated with hyperglycemia and hyperinsulinemia[21,22]. Induction of lipogenic genes is under the combined actions of sterol regulatory element binding protein-1c (SREBP-1c) in response to insulin, and ChREBP (carbohydrate responsive element binding protein) in response to glucose[23,24]. These events also result in a shift in cellular metabolism from lipid oxidation to triglyceride esterification, thereby increasing the production of liver triglycerides[12,25].
MODULATION OF HEPATIC LIPID METABOLISM BY DIETARY FATTY ACIDS
It is widely known that the fatty acid composition of the diet is an important factor capable of influencing hepatic lipid metabolism. Indeed, dietary fatty acids are able to regulate various metabolic pathways involved in lipid metabolism mainly through a fine modulation of gene transcription of specific enzymes[16,26]. Since the liver plays a key role in lipid metabolism, dietary fats and their oxidized metabolites may not only influence the pathogenesis of liver diseases[27-31], but may also prevent and/or reverse disease manifestations[32]. In particular, it has been reported that n-3 polyunsaturated fatty acids (PUFA) are able to limit triglyceride deposition in the liver[33,34], whereas a diet deficient in n-3 PUFA with a high n-6/n-3 ratio could induce fatty liver[28] and chronic diseases[35-37].
Modulation of hepatic lipid metabolism by fatty acids is quite complex, involving a sequence of molecular events which are interdependent and cross-regulated. Because of the complexity of this topic, in the present review we focus our attention on dietary fat modulation of hepatic fatty acid synthesis, a pathway which plays a key role in the pathogenesis of liver fat accumulation. It is known from the literature that dietary PUFA of the n-3 and n-6 series are potent inhibitors of hepatic lipogenesis[16]. However, most of these studies investigated the variations in the activity and in the expression of the cytosolic lipogenic enzymes (ACC and FAS).
In recent years, attention has also been gradually directed towards the effects of dietary PUFA of the n-3 and n-6 series on the activity of the mitochondrial CIC, which, as stated before, transports citrate outside mitochondria for cytosolic fatty acid biosynthesis. It has been found that PUFA significantly decrease the activity and the expression of the mitochondrial CIC[38-43]. Very interestingly, parallel reductions in the activities of mitochondrial CIC and of cytosolic lipogenic enzymes were found, thereby highlighting a close coordination between mitochondrial and cytosolic reactions[38-44]. In contrast, a diet enriched in monounsaturated fatty acids (MUFA) or saturated fatty acids (SFA) did not exert any appreciable effect on mitochondrial CIC activity and expression, and therefore did not influence de novo fatty acid synthesis[41,42]. This mitochondrial carrier, therefore, acts as a sensor for changes occurring in hepatic lipogenesis[39-44] which, in turn, may influence fat deposition in liver.
DIETARY FATTY ACIDS AND PREVENTION OF HEPATIC STEATOSIS: NEW INSIGHTS FROM KRILL AND PINE NUT OIL
In recent years, the use of dietary supplements has rapidly increased. For instance, krill oil, a novel dietary supplement of n-3 PUFA, has become increasingly popular as a food supplement during the last decade. This oil is extracted from Antarctic krill (Euphausia superba), a shrimp-like zooplankton at the bottom of the food chain. Krill oil contains two n-3 PUFA, eicosapentaenoic acid (EPA, 20:5) and docosahexanoic acid (DHA, 22:6), in amounts similar to those present in fish oil. However, these long-chain n-3 fatty acids are present in krill oil in the form of phospholipids rather than triglycerides. Furthermore, the ratio of EPA to DHA is different in the two oils, with EPA prevailing in krill oil and DHA prevailing in fish oil[45,46]. The health-promoting effects of krill oil have been reported both in humans and in animal models by several authors[47-50]. Moreover, a higher potency of krill oil in comparison to fish oil has also been proposed[43,51]. This may be biologically and therapeutically significant, since it has been found that krill oil supplementation showed beneficial effects on hepatomegaly and hepatic steatosis in mice fed a high-fat diet (Table 1). Indeed, krill oil supplementation of the diet caused a significant reduction in liver weight (hepatomegaly) and total liver fat (steatosis)[42,50]. Recently, investigation of the molecular mechanisms responsible for the action of krill oil revealed that the beneficial effects of this fat were due to a favourable combination of several elements[42]. First, diet supplementation with 2.5% krill oil in rats fed a high-fat diet reduced the activity and expression of mitochondrial CIC, thereby decreasing the amount of substrate available for hepatic fatty acid synthesis. The concomitant and concerted reduction of the lipogenic enzymes ACC and FAS resulted in a strong inhibition of hepatic fatty acid synthesis. A similar effect was also observed when animals were fed with a standard diet, suggesting that krill oil has an intrinsic capability to reduce hepatic lipogenesis[43]. Second, besides the inhibition of de novo lipogenesis, a marked increase in fatty acid oxidation was observed in animals fed with a diet enriched in krill oil. Third, dietary krill oil was also able to retain efficient mitochondrial oxidative phosphorylation in treated rats, thus preventing the possible uncoupling effects of a high-fat diet. Overall, krill oil stimulated the catabolization of excess fat introduced by a hypercaloric diet, while inhibiting de novo fatty acid synthesis and therefore preventing the onset of fatty liver.
Table 1.
Use of dietary supplement in the prevention of hepatic steatosis
| Oil source | Oil fatty acid composition (%) | Experimental design | Results | Ref. | |
| Krill | C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 C20:5 C22:5 C22:6 | 2.5 18.2 2.8 25.8 54.4 4.9 5.3 2.3 3.0 | C57BL/6 mice fed for 8 wk with a high-fat diet containing 1.25%-5% krill oil | ↓ Hepatomegaly ↓ Hepatic steatosis ↓ De novo lipogenesis ↓ Blood glucose | Tandy et al[50] |
| Wistar rats fed for 6 wk with a diet containing 2.5% krill oil | ↓ Hepatic steatosis ↓ De novo lipogenesis ↓ Haematic triglycerides ↓ Haematic cholesterol | Ferramosca et al[43] | |||
| Sprague-Dawley rats fed for 12 wk with a high-fat diet containing 2.5% krill oil | ↓ Hepatic steatosis ↓ De novo lipogenesis ↑ Fatty acid oxidation ↓ Mitochondrial uncoupling ↓ Haematic glucose ↓ Haematic insulin ↓ Haematic triglycerides | Ferramosca et al[42] | |||
| Pine nut | C16:0 C18:0 C18:1 C18:2 C18:3 C18:3 (5,9,12) | 8.0 2.2 23.2 51.1 2.1 8.8 | Mice fed for 8 wk with a diet containing 7.5% pine nut oil | ↓ Liver weight ↓ Hepatic steatosis ↓ De novo lipogenesis ↓ Haematic triglycerides ↓ Haematic cholesterol | Ferramosca et al[40] |
It is also important to underline that surplus of energy supplied by fat often leads to reduced tissue utilization of glucose, thus causing hyperglycemia and hyperinsulinemia[52]. Very interestingly, krill oil was also able to reverse the increase in the levels of blood glucose and insulin, normally observed in steatotic animals, thus preventing insulin resistance[42]. On the other hand, lower levels of triglycerides were found in plasma of animals fed with krill oil in comparison to those detected in rats fed a high-fat diet, and this may also be significant in preventing cardiovascular diseases. All these results suggest that krill oil has the ability to improve lipid and glucose metabolism and highlight the possible protective effects of krill oil against hepatic steatosis.
In the last few years, vegetable oils extracted from the seeds of some conifers[53] have been also under investigation for their use as dietary supplements[54-56]. The oil from the seeds of Pinus koraiensis is a dietary fat that contains, along with various fatty acids, pinolenic acid or all-cis-5,9,12-octadecatrienoic acid. This is a quite unusual n-6 PUFA which is characterized by polymethylene-interrupted double bonds. Preliminary studies carried out in rats indicated that pine nut oil exerted some beneficial effects on lipid metabolism, such as a decrease in plasma triglycerides and in circulating very-low-density lipoprotein[54,56]. Further studies demonstrated that diet supplementation with pine nut oil caused a significant reduction in liver weight and liver lipids[40]. These results are noteworthy because this dietary fat might be of interest in the case of hepatic steatosis (Table 1). A concomitant reduction in the mitochondrial CIC activity and in the cytosolic ACC and FAS activities was observed in animals fed pine nut oil[40]. However, a similar decrease in de novo fatty acid synthesis was also found in control mice which were fed with a diet enriched with maize oil. This latter diet has a fatty acid composition similar to that of the pine nut oil diet, except for the absence of pinolenic acid. Therefore, the specific capability of decreasing hepatic and plasma lipids, shown by pine nut oil, is probably due to pinolenic acid, or to some of its possible metabolites[40].
DIETARY FATTY ACIDS AND DEVELOPMENT OF HEPATIC STEATOSIS
Fatty liver is diet-inducible in rodent animal models, in which high-fat diets are able to cause an increase in the liver fat levels. Indeed, an increase in the level of liver lipids was observed in rats fed for 12 wk with a diet containing a high content of fat (35% lard)[42] (Table 2). It must be underlined that the approximate fatty acid profile of the high-fat diet used in this study was kept low in PUFA, with the aim of preventing the inhibitory effect of hepatic fatty acid synthesis by high levels of these unsaturated fatty acids. The excess dietary fat anyhow inhibited hepatic lipogenesis at the beginning of dietary treatment. This inhibition progressively decreased over time and was completely abolished at longer feeding times. The high level of triglycerides found in the liver at the beginning of this dietary treatment was therefore not due to an increased fatty acid synthesis, since this anabolic pathway was inhibited at that time. Interestingly, a decrease in fatty acid oxidation, as well as a strong decrease in mitochondrial respiratory efficiency, was clearly observed in animals fed a high-fat diet[42]. This last observation suggests that the excess of fat in the diet most probably induced a partial uncoupling between respiration and phosphorylation in the mitochondria[57]. A concomitant increase in the plasma levels of glucose and insulin was also observed in animals fed a high-fat diet[42].
Table 2.
Role of dietary fat in the development of hepatic steatosis
| Dietary fat | Fatty acid composition (%) | Experimental design | Results | Ref. | |
| Lard | C14:0 C16:0 C18:0 C18:1 C18:2 | 0.50 8.70 4.30 15.80 3.50 | Sprague-Dawley rats fed for 12 wk with a high-fat diet (35% fat) | ↑ Hepatic steatosis ↓ Fatty acid oxidation ↑ Haematic triglycerides ↑ Mitochondrial uncoupling ↑ Haematic insulin ↑ Haematic glucose | Ferramosca et al[42] |
| CLA c-9, t-11 CLA t-10, c-12 | 0.004 0.40 | C57Bl/6J mice fed were fed for 4 wk with a diet containing 0.4% CLA | ↑ Hepatic steatosis ↑ De novo lipogenesis ↑ Haematic insulin | Clément et al[64] | |
| CLA | C16:0 C18:0 C18:1 C18:2 CLA c-9, t-11/ t-9, c-11 CLA t-10, c-12 CLA c-9, c-11/c10, c12 CLA t-9, t-11/t10, t12 | 5.91 0.60 5.27 1.22 0.49 0.51 0.03 0.02 | C57BL/6J mice were fed for 21 days with a diet containing 1.5% CLA | ↑ Hepatomegaly ↑ Hepatic steatosis ↑ De novo lipogenesis ↑ Fatty acid oxidation | Takahashi et al[65] |
| CLA t-10, c-12 | 1.00 | C57BL/6J mice fed for 4 wk with a diet supplemented with 1% CLA | ↑ Liver weight ↓ Haematic triglycerides ↓ Haematic FFA ↑ Fatty acid oxidation | Degrace et al[68] | |
| C16:0 C16:1 C18:0 C18:1 C18:2 CLA c-9, t-11 CLA t-10, c-12 CLA c-9, c-11/c10, c12 CLA t-9, t-11/t10, t12 | 5.94 0.01 0.60 5.30 1.27 0.49 0.51 0.03 0.02 | ICR mice fed for 22 d with a diet containing 1.0% CLA | ↑ Liver weight ↑ Hepatic steatosis ↑ De novo lipogenesis ↑ Fatty acid oxidation ↑ Haematic insulin | Ide[66] | |
| C16:0 C18:0 C18:1 C18:2 C18:3 CLA c-9, t-11 CLA t-10, c-12 | 12.00 2.70 42.50 30.50 2.90 3.50 3.70 | ICR mice fed for 16 wk with a diet containing 1% CLA | ↑ Liver weight ↑ Hepatic steatosis ↑ De novo lipogenesis ↑ Fatty acid oxidation ↓ Haematic triglycerides ↓ Haematic FFA | Ferramosca et al[44] | |
| C16:0 C18:0 C18:1 C18:2 C18:3 CLA c-9, t-11 CLA t-10, c-12 | 12.00 2.70 42.50 30.50 2.90 3.50 3.70 | ICR mice fed for 16 wk with a diet containing 1% CLA | ↑ Liver weight ↑ Hepatic steatosis ↑ De novo lipogenesis ↑ Fatty acid oxidation ↓ Haematic triglycerides ↓ Haematic cholesterol ↑ Haematic insulin | Ferramosca et al[39] | |
CLA: Conjugated linoleic acids.
In the last decade, the attention of some authors has been focused on the fatty acid composition of the diet in the induction of hepatic steatosis. In this context, a recent review reports that an increase in free fatty acids, especially SFA, may play an important role in the development of hepatic steatosis[29]. It has been demonstrated that SFA caused liver dysfunction by promoting endoplasmic reticulum stress and apoptosis[58-60]. In contrast to SFA, an increase in MUFA induced steatotic liver, but did not initiate apoptosis[61].
Conjugated linoleic acids (CLA) have also been linked to the development of hepatic steatosis. CLA is the acronym for a class of positional and geometric isomers of linoleic acid[62]. These compounds are naturally present in food derived from ruminant animals, such as bovine and ovine meat and dairy products. The main CLA isomer in natural products is the cis-9,trans-11-octadecadienoic acid, but the commercially available CLA, currently used as a food supplement, contains a 1:1 mixture of this isomer and the trans-10,cis-12 isomer. Several authors have indicated that CLA has beneficial effects in the case of cardiovascular diseases, obesity and diabetes[63]. These beneficial effects, however, are in some instances associated with adverse effects, such as liver steatosis[64].
Several studies (Table 2) suggested that de novo fatty acid synthesis may play a role in the onset of hepatic steatosis produced by CLA administration[44,65,66]. Interestingly, in CLA-fed mice, a time-dependent increase in the enzymatic activities involved in hepatic lipogenesis was clearly found. Indeed, at the 16th week of CLA feeding, an approximate doubling of the activities of mitochondrial CIC and of cytosolic lipogenic enzymes (ACC and FAS) was detected[39,44]. It is important to underline that, in the first period of CLA feeding, liver enlargement and hepatic triglyceride accumulation occurred independently of the fatty acid synthesis stimulation. At longer times (weeks 12-16), the level of hepatic triglycerides in CLA-fed mice increased dramatically. The concomitant strong increase in the levels of plasma insulin suggested that this hormone, possibly in addition to other factors, could play an important role in the development of hepatic steatosis after longer durations of dietary treatment[39].
Several authors reported that trans-10,cis-12 CLA was the isomer responsible for the development of fatty liver in mice in which a loss of adipose tissue was concomitantly observed[64,67,68]. However, the mechanisms by which the liver becomes steatotic in response to trans-10, cis-12 CLA are not well understood and appear to be puzzling since this isomer also induces a concomitant increase in cellular fatty acid oxidation[68].
“CASE” OF OLIVE OIL
Olive oil, a basic component of the Mediterranean diet, mainly contains oleic acid, a MUFA fatty acid of the n-9 series. One of the most intriguing aspects regarding olive oil is its effect on hepatic lipid metabolism. In some studies (Table 3) carried out in rodents, an olive oil-enriched diet induced fat accumulation in the liver[41,69-72]. Furthermore, in olive oil-treated animals, an increase in hepatic lipogenesis was surprisingly found[71,72]. However, the animals used as control group in these studies were fed with a diet enriched in PUFA. Therefore, the observed increase in hepatic lipogenesis in olive oil-fed animals was only apparent and probably due to the comparison with the PUFA-enriched diet fed to control animals.
Table 3.
Effect of olive oil on liver lipid profile
| Fatty acid composition (%) | Experimental design | Results | Ref. | |
| C16:0 C16:1 C17:0 C18:0 C18:1 C18:2 C18:3 C20:0 C20:1 C24:0 | 11.80 0.90 0.40 2.80 79.20 3.50 0.60 0.30 0.20 0.40 | Wistar rats fed for 12 wk with a diet containing 10% olive oil | ↑ Hepatic lipids | Ruiz-Gutiérrez et al[69] |
| C16:0 C16:1 C17:0 C18:0 C18:1 C18:2 C18:3 C20:0 C20:1 C24:0 | 11.79 0.86 0.37 2.79 79.22 3.45 0.60 0.28 0.20 0.44 | Spontaneously hypertensive rats fed for 12 wk with a diet containing 10% of energy as olive oil | ↑ Hepatic lipids | Perona et al[70] |
| C16:0 C16:1 C18:0 C18:1 C18:2 C18:3 C20:0 | 11.87 0.94 2.92 77.26 5.69 0.50 0.39 | Wistar rats fed for 4 wk with a diet containing 40% of energy as olive oil | ↑ Hepatic lipids ↑ De novo lipogenesis | Portillo et al[71] |
| C16:0 C18:0 C18:1 C18:2 C18:3 | 10.70 2.00 74.80 10.40 0.70 | Wistar rats fed for 6 wk with a diet containing 20% olive oil | ↑ Hepatic lipids ↑ De novo lipogenesis | Takeuchi et al[72] |
| C16:0 C18:1 C18:2 | 11.29 71.26 9.76 | Sprague-Dawley rats fed for 2 mo with a methionine choline-deficient diet containing olive oil (0.45 mg/g rat weight) | ↓ Hepatic steatosis | Hussein et al[73] |
| C16:0 C18:0 C18:1 C18:2 C18:3 | 12.10 3.00 27.40 51.90 2.70 | ICR mice fed for 8 wk with a diet containing 7.5% olive oil | ↑ Hepatic lipids ↓ Fatty acid oxidation | Ferramosca et al[41] |
Nevertheless, the molecular mechanisms of fat accumulation in the liver of olive oil fed animals are not clear. In the organism, the liver plays a fundamental role in lipid metabolism, because it is involved in many different processes, such as fatty acid uptake, storage, conversion, oxidation, synthesis and secretion. Therefore, a clear definition of the molecular events leading to lipid accumulation in the liver, consequent to olive oil administration, is quite difficult. However, it has been proposed that the increase in the hepatic triglyceride content of olive oil-fed mice was due to an impairment of mitochondrial fatty acid oxidation[41].
On the other hand, an interesting study[73], carried out in rats with NAFLD, demonstrated that olive oil decreased the accumulation of liver triglycerides. In particular, it has been suggested that olive oil may improve insulin resistance, increase the release of triglyceride from liver and decrease the lipolytic flux from peripheral adipose tissue in steatotic animals[73]. Further studies, carried out in humans, demonstrated that MUFA were able to increase lipid oxidation and to decrease insulin resistance, suggesting that olive oil should be included in the diet of NAFLD patients[74,75].
SPECIFIC COMBINATIONS OF PUFA CAN MODULATE HEPATIC STEATOSIS
Some attempts have been made to overcome the adverse effects of CLA, such as fatty liver, by using appropriate mixtures of CLA and other dietary fatty acids. Nakanishi et al[76] found that the addition of gamma-linolenic acid to a CLA-enriched diet was able to prevent fatty liver in mice, even if the antiobesity properties of CLA were only partially retained. Another study[77] demonstrated that 0.5% supplementation of DHA to the CLA diet attenuated CLA-induced fatty liver through the reduction of hepatic fatty acid synthesis. In agreement with these results, Ide[66] demonstrated that different amounts of fish oil added to a CLA-enriched diet downregulated de novo lipogenesis in a dose-dependent manner, concomitantly reducing hepatic triglyceride levels. Indeed, both dietary CLA and fish oil strongly affect hepatic lipogenic activities: CLA enhances, whereas fish oil inhibits the activities and the expression of the enzymes involved in hepatic lipogenesis[43,44]. Therefore, the simultaneous ingestion of fish oil and CLA may represent a “dietary trick” to retain the positive effects exerted by CLA, such as its anti-obesity property, while avoiding the negative consequences mainly consisting of excessive fat deposition in the liver.
In line with this concept, a further study[39] investigated the effects of a dietary combination of CLA and pine nut oil on lipid metabolism in mice. Previous studies demonstrated that CLA greatly increased de novo fatty acid synthesis in mouse hepatocytes, thus leading to hepatic steatosis[44]. In contrast, pine nut oil decreased liver lipid concentration[40]. Starting with these observations, it has been found that the co-administration of CLA and pine nut oil in the diet exerted a series of positive effects in treated animals: (1) the CLA-mediated body fat reduction was preserved; (2) the onset of hepatic steatosis was effectively prevented; and (3) the liver and plasma lipid content was normalized. The detailed analysis of metabolic changes occurring in mice under this dietary treatment revealed that the enzymatic activities involved in fatty acid synthesis had time-dependent biphasic behavior. In fact, hepatic lipogenesis showed a biphasic trend in CLA + pine nut oil-fed animals, consisting of a moderate increase within the first 6-8 wk of dietary treatment, followed by a progressive decrease from the 8th week onward. A strong decrease in hepatic fatty synthesis was indeed detected at the 16th week of feeding. Furthermore, whereas a sharp increase in plasma insulin levels occurred in CLA-fed animals at the 8th week, insulinemia remained stable in CLA + pine nut oil-fed mice. It was also found that a CLA + pine nut oil diet positively influenced plasma lipid levels. In fact, this dietary association reinforced the capability of CLA in decreasing plasma triglyceride levels, reducing at the same time plasma levels of cholesterol and phospholipids. The healthy beneficial effects promoted by a CLA + pine nut oil diet are probably due to the peculiar fatty acid composition of the mixture. In fact, the CLA + pine nut oil diet has a lower amount of SFA and MUFA and a higher amount of PUFA in comparison to the CLA diet. Among PUFA, the specific presence of 1.12% pinolenic acid may have a fundamental impact on the observed hypolipidemic effects.
CONCLUSION
NAFLD is characterized by abnormal fat deposition in the liver, where lipids are mainly stored as triglycerides. Hepatic fat accumulation results from an imbalance between lipid supply (uptake or de novo lipogenesis) and lipid clearance (fatty-acid oxidation or triglyceride-rich lipoprotein secretion). Among the mechanisms involved in triglyceride accumulation, uptake of fatty acids consequent to adipose tissue lipolysis and de novo lipogenesis seem to be the major sources of lipid in the steatotic liver[13].
In recent years, several studies carried out in animal models focused their attention on the role of dietary fats in the modulation of de novo lipogenesis, which plays a significant role in the pathogenesis of NAFLD. It has been demonstrated that a high-fat diet is able to induce a condition of hepatic steatosis. A similar effect was observed after the dietary administration of CLA which, at the same time, are able to strongly prevent fat accumulation in adipose tissue. While the effects of olive oil on hepatic lipid content are not completely clear, novel dietary supplements, such as krill oil or pine nut oil, seem to have a protective effect against hepatic steatosis. Nevertheless, further studies in humans are needed to ascertain whether the consumption of these dietary fats may be helpful in NAFLD patients.
Footnotes
P- Reviewers: Akcam M, Caprio S S- Editor: Zhai HH L- Editor: Cant MR E- Editor: Wu HL
References
- 1.Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560–578. doi: 10.1007/s10620-009-1081-0. [DOI] [PubMed] [Google Scholar]
- 2.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 4.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 6.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagano C, Soardo G, Esposito W, Fallo F, Basan L, Donnini D, Federspil G, Sechi LA, Vettor R. Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur J Endocrinol. 2005;152:113–118. doi: 10.1530/eje.1.01821. [DOI] [PubMed] [Google Scholar]
- 8.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 9.Serviddio G, Sastre J, Bellanti F, Viña J, Vendemiale G, Altomare E. Mitochondrial involvement in non-alcoholic steatohepatitis. Mol Aspects Med. 2008;29:22–35. doi: 10.1016/j.mam.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postic C, Girard J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 2008;34:643–648. doi: 10.1016/S1262-3636(08)74599-3. [DOI] [PubMed] [Google Scholar]
- 13.Ferré P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12 Suppl 2:83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 14.Girard J, Perdereau D, Foufelle F, Prip-Buus C, Ferré P. Regulation of lipogenic enzyme gene expression by nutrients and hormones. FASEB J. 1994;8:36–42. doi: 10.1096/fasebj.8.1.7905448. [DOI] [PubMed] [Google Scholar]
- 15.Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45:199–214. doi: 10.3109/10409231003667500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jump DB. Fatty acid regulation of hepatic lipid metabolism. Curr Opin Clin Nutr Metab Care. 2011;14:115–120. doi: 10.1097/MCO.0b013e328342991c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lou-Bonafonte JM, Arnal C, Osada J. New genes involved in hepatic steatosis. Curr Opin Lipidol. 2011;22:159–164. doi: 10.1097/MOL.0b013e3283462288. [DOI] [PubMed] [Google Scholar]
- 18.Barrows BR, Timlin MT, Parks EJ. Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL-triacylglycerol under two different feeding regimens. Diabetes. 2005;54:2668–2673. doi: 10.2337/diabetes.54.9.2668. [DOI] [PubMed] [Google Scholar]
- 19.Palmieri F, Bisaccia F, Iacobazzi V, Indiveri C, Zara V. Mitochondrial substrate carriers. Biochim Biophys Acta. 1992;1101:223–227. [PubMed] [Google Scholar]
- 20.Watson JA, Lowenstein JM. Citrate and the conversion of carbohydrate into fat. Fatty acid synthesis by a combination of cytoplasm and mitochondria. J Biol Chem. 1970;245:5993–6002. [PubMed] [Google Scholar]
- 21.Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foufelle F, Ferré P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem J. 2002;366:377–391. doi: 10.1042/BJ20020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flannery C, Dufour S, Rabøl R, Shulman GI, Petersen KF. Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly. Diabetes. 2012;61:2711–2717. doi: 10.2337/db12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 2005;87:81–86. doi: 10.1016/j.biochi.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 2008;138:1039–1046. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen P, Leray V, Diez M, Serisier S, Le Bloc’h J, Siliart B, Dumon H. Liver lipid metabolism. J Anim Physiol Anim Nutr (Berl) 2008;92:272–283. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Gura KM, Puder M. Omega-3 fatty acids and liver disease. Hepatology. 2007;45:841–845. doi: 10.1002/hep.21645. [DOI] [PubMed] [Google Scholar]
- 28.El-Badry AM, Graf R, Clavien PA. Omega 3 - Omega 6: What is right for the liver? J Hepatol. 2007;47:718–725. doi: 10.1016/j.jhep.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res. 2013;52:165–174. doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, Zhang R, McIntyre TM, Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santoro N, Caprio S, Giannini C, Kim G, Kursawe R, Pierpont B, Shaw MM, Feldstein AE. Oxidized Fatty acids: a potential pathogenic link between Fatty liver and type 2 diabetes in obese adolescents? Antioxid Redox Signal. 2014;20:383–389. doi: 10.1089/ars.2013.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masterton GS, Plevris JN, Hayes PC. Review article: omega-3 fatty acids - a promising novel therapy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;31:679–692. doi: 10.1111/j.1365-2036.2010.04230.x. [DOI] [PubMed] [Google Scholar]
- 33.Xin YN, Xuan SY, Zhang JH, Zheng MH, Guan HS. Omega-3 polyunsaturated fatty acids: a specific liver drug for non-alcoholic fatty liver disease (NAFLD) Med Hypotheses. 2008;71:820–821. doi: 10.1016/j.mehy.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Di Minno MN, Russolillo A, Lupoli R, Ambrosino P, Di Minno A, Tarantino G. Omega-3 fatty acids for the treatment of non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18:5839–5847. doi: 10.3748/wjg.v18.i41.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen HS. Dietary essential fatty acids and in vivo prostaglandin production in mammals. World Rev Nutr Diet. 1983;42:102–134. doi: 10.1159/000408352. [DOI] [PubMed] [Google Scholar]
- 36.Zampelas A, Paschos G, Rallidis L, Yiannakouris N. Linoleic acid to alpha-linolenic acid ratio. From clinical trials to inflammatory markers of coronary artery disease. World Rev Nutr Diet. 2003;92:92–108. doi: 10.1159/000073795. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Zara V, Giudetti AM, Siculella L, Palmieri F, Gnoni GV. Covariance of tricarboxylate carrier activity and lipogenesis in liver of polyunsaturated fatty acid (n-6) fed rats. Eur J Biochem. 2001;268:5734–5739. doi: 10.1046/j.0014-2956.2001.02508.x. [DOI] [PubMed] [Google Scholar]
- 39.Ferramosca A, Savy V, Conte L, Zara V. Dietary combination of conjugated linoleic acid (CLA) and pine nut oil prevents CLA-induced fatty liver in mice. J Agric Food Chem. 2008;56:8148–8158. doi: 10.1021/jf8010728. [DOI] [PubMed] [Google Scholar]
- 40.Ferramosca A, Savy V, Einherand AWC, Zara V. Pinus koraiensis seed oil (PinnoThinTM) supplementation reduces body weight gain and lipid concentration of liver and plasma in mice. J Animal Feed Sci. 2008;17:621–630. [Google Scholar]
- 41.Ferramosca A, Savy V, Zara V. Olive oil increases the hepatic triacylglycerol content in mice by a distinct influence on the synthesis and oxidation of fatty acids. Biosci Biotechnol Biochem. 2008;72:62–69. doi: 10.1271/bbb.70369. [DOI] [PubMed] [Google Scholar]
- 42.Ferramosca A, Conte A, Burri L, Berge K, De Nuccio F, Giudetti AM, Zara V. A krill oil supplemented diet suppresses hepatic steatosis in high-fat fed rats. PLoS One. 2012;7:e38797. doi: 10.1371/journal.pone.0038797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferramosca A, Conte L, Zara V. A krill oil supplemented diet reduces the activities of the mitochondrial tricarboxylate carrier and of the cytosolic lipogenic enzymes in rats. J Anim Physiol Anim Nutr (Berl) 2012;96:295–306. doi: 10.1111/j.1439-0396.2011.01135.x. [DOI] [PubMed] [Google Scholar]
- 44.Ferramosca A, Savy V, Conte L, Colombo S, Einerhand AW, Zara V. Conjugated linoleic acid and hepatic lipogenesis in mouse: role of the mitochondrial citrate carrier. J Lipid Res. 2006;47:1994–2003. doi: 10.1194/jlr.M600138-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Kolakowska A, Kolakowski E, Szczygielski M. Winter season krill (Euphausia superba Dana) as a source of n-3 polyunsaturated fatty acids. Food/Nahrung. 1994;38:128–134. [Google Scholar]
- 46.Burri L, Hoem N, Banni S, Berge K. Marine omega-3 phospholipids: metabolism and biological activities. Int J Mol Sci. 2012;13:15401–15419. doi: 10.3390/ijms131115401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bunea R, El Farrah K, Deutsch L. Evaluation of the effects of Neptune Krill Oil on the clinical course of hyperlipidemia. Altern Med Rev. 2004;9:420–428. [PubMed] [Google Scholar]
- 48.Deutsch L. Evaluation of the effect of Neptune Krill Oil on chronic inflammation and arthritic symptoms. J Am Coll Nutr. 2007;26:39–48. doi: 10.1080/07315724.2007.10719584. [DOI] [PubMed] [Google Scholar]
- 49.Zhu JJ, Shi JH, Qian WB, Cai ZZ, Li D. Effects of krill oil on serum lipids of hyperlipidemic rats and human SW480 cells. Lipids Health Dis. 2008;7:30. doi: 10.1186/1476-511X-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tandy S, Chung RW, Wat E, Kamili A, Berge K, Griinari M, Cohn JS. Dietary krill oil supplementation reduces hepatic steatosis, glycemia, and hypercholesterolemia in high-fat-fed mice. J Agric Food Chem. 2009;57:9339–9345. doi: 10.1021/jf9016042. [DOI] [PubMed] [Google Scholar]
- 51.Burri L, Berge K, Wibrand K, Berge RK, Barger JL. Differential effects of krill oil and fish oil on the hepatic transcriptome in mice. Front Genet. 2011;2:45. doi: 10.3389/fgene.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikemoto S, Takahashi M, Tsunoda N, Maruyama K, Itakura H, Ezaki O. High-fat diet-induced hyperglycemia and obesity in mice: differential effects of dietary oils. Metabolism. 1996;45:1539–1546. doi: 10.1016/s0026-0495(96)90185-7. [DOI] [PubMed] [Google Scholar]
- 53.Wolff RL, Pédrono F, Pasquier E, Marpeau AM. General characteristics of Pinus spp. seed fatty acid compositions, and importance of delta5-olefinic acids in the taxonomy and phylogeny of the genus. Lipids. 2000;35:1–22. doi: 10.1007/s11745-000-0489-y. [DOI] [PubMed] [Google Scholar]
- 54.Sugano M, Ikeda I, Wakamatsu K, Oka T. Influence of Korean pine (Pinus koraiensis)-seed oil containing cis-5,cis-9,cis-12-octadecatrienoic acid on polyunsaturated fatty acid metabolism, eicosanoid production and blood pressure of rats. Br J Nutr. 1994;72:775–783. doi: 10.1079/bjn19940079. [DOI] [PubMed] [Google Scholar]
- 55.Matsuo N, Osada K, Kodama T, Lim BO, Nakao A, Yamada K, Sugano M. Effects of gamma-linolenic acid and its positional isomer pinolenic acid on immune parameters of brown-Norway rats. Prostaglandins Leukot Essent Fatty Acids. 1996;55:223–229. doi: 10.1016/s0952-3278(96)90002-2. [DOI] [PubMed] [Google Scholar]
- 56.Asset G, Staels B, Wolff RL, Baugé E, Madj Z, Fruchart JC, Dallongeville J. Effects of Pinus pinaster and Pinus koraiensis seed oil supplementation on lipoprotein metabolism in the rat. Lipids. 1999;34:39–44. doi: 10.1007/s11745-999-335-2. [DOI] [PubMed] [Google Scholar]
- 57.Vial G, Dubouchaud H, Couturier K, Cottet-Rousselle C, Taleux N, Athias A, Galinier A, Casteilla L, Leverve XM. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J Hepatol. 2011;54:348–356. doi: 10.1016/j.jhep.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 58.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 59.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 60.Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298:E1027–E1035. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly GS. Conjugated linoleic acid: a review. Altern Med Rev. 2001;6:367–382. [PubMed] [Google Scholar]
- 63.Vyas D, Kadegowda AK, Erdman RA. Dietary conjugated linoleic Acid and hepatic steatosis: species-specific effects on liver and adipose lipid metabolism and gene expression. J Nutr Metab. 2012;2012:932928. doi: 10.1155/2012/932928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clément L, Poirier H, Niot I, Bocher V, Guerre-Millo M, Krief S, Staels B, Besnard P. Dietary trans-10,cis-12 conjugated linoleic acid induces hyperinsulinemia and fatty liver in the mouse. J Lipid Res. 2002;43:1400–1409. doi: 10.1194/jlr.m20008-jlr200. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi Y, Kushiro M, Shinohara K, Ide T. Activity and mRNA levels of enzymes involved in hepatic fatty acid synthesis and oxidation in mice fed conjugated linoleic acid. Biochim Biophys Acta. 2003;1631:265–273. doi: 10.1016/s1388-1981(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 66.Ide T. Interaction of fish oil and conjugated linoleic acid in affecting hepatic activity of lipogenic enzymes and gene expression in liver and adipose tissue. Diabetes. 2005;54:412–423. doi: 10.2337/diabetes.54.2.412. [DOI] [PubMed] [Google Scholar]
- 67.Park Y, Storkson JM, Albright KJ, Liu W, Pariza MW. Evidence that the trans-10,cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids. 1999;34:235–241. doi: 10.1007/s11745-999-0358-8. [DOI] [PubMed] [Google Scholar]
- 68.Degrace P, Demizieux L, Gresti J, Chardigny JM, Sébédio JL, Clouet P. Hepatic steatosis is not due to impaired fatty acid oxidation capacities in C57BL/6J mice fed the conjugated trans-10,cis-12-isomer of linoleic acid. J Nutr. 2004;134:861–867. doi: 10.1093/jn/134.4.861. [DOI] [PubMed] [Google Scholar]
- 69.Ruiz-Gutiérrez V, Pérez-Espinosa A, Vázquez CM, Santa-María C. Effects of dietary fats (fish, olive and high-oleic-acid sunflower oils) on lipid composition and antioxidant enzymes in rat liver. Br J Nutr. 1999;82:233–241. [PubMed] [Google Scholar]
- 70.Perona JS, Ruiz-Gutiérrez V. Effect of two high-oleic oils on the liver lipid composition of spontaneously hypertensive rats. Life Sci. 2000;66:521–531. doi: 10.1016/s0024-3205(99)00622-0. [DOI] [PubMed] [Google Scholar]
- 71.Portillo MP, Chávarri M, Durán D, Rodríguez VM, Macarulla MT. Differential effects of diets that provide different lipid sources on hepatic lipogenic activities in rats under ad libitum or restricted feeding. Nutrition. 2001;17:467–473. doi: 10.1016/s0899-9007(01)00513-5. [DOI] [PubMed] [Google Scholar]
- 72.Takeuchi H, Nakamoto T, Mori Y, Kawakami M, Mabuchi H, Ohishi Y, Ichikawa N, Koike A, Masuda K. Comparative effects of dietary fat types on hepatic enzyme activities related to the synthesis and oxidation of fatty acid and to lipogenesis in rats. Biosci Biotechnol Biochem. 2001;65:1748–1754. doi: 10.1271/bbb.65.1748. [DOI] [PubMed] [Google Scholar]
- 73.Hussein O, Grosovski M, Lasri E, Svalb S, Ravid U, Assy N. Monounsaturated fat decreases hepatic lipid content in non-alcoholic fatty liver disease in rats. World J Gastroenterol. 2007;13:361–368. doi: 10.3748/wjg.v13.i3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soriguer F, Morcillo S, Cardona F, Rojo-Martínez G, de la Cruz Almaráz M, Ruiz de Adana Mde L, Olveira G, Tinahones F, Esteva I. Pro12Ala polymorphism of the PPARG2 gene is associated with type 2 diabetes mellitus and peripheral insulin sensitivity in a population with a high intake of oleic acid. J Nutr. 2006;136:2325–2330. doi: 10.1093/jn/136.9.2325. [DOI] [PubMed] [Google Scholar]
- 75.Assy N, Nassar F, Nasser G, Grosovski M. Olive oil consumption and non-alcoholic fatty liver disease. World J Gastroenterol. 2009;15:1809–1815. doi: 10.3748/wjg.15.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakanishi T, Oikawa D, Koutoku T, Hirakawa H, Kido Y, Tachibana T, Furuse M. Gamma-linolenic acid prevents conjugated linoleic acid-induced fatty liver in mice. Nutrition. 2004;20:390–393. doi: 10.1016/j.nut.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 77.Yanagita T, Wang YM, Nagao K, Ujino Y, Inoue N. Conjugated linoleic acid-induced fatty liver can be attenuated by combination with docosahexaenoic acid in C57BL/6N mice. J Agric Food Chem. 2005;53:9629–9633. doi: 10.1021/jf052203i. [DOI] [PubMed] [Google Scholar]


