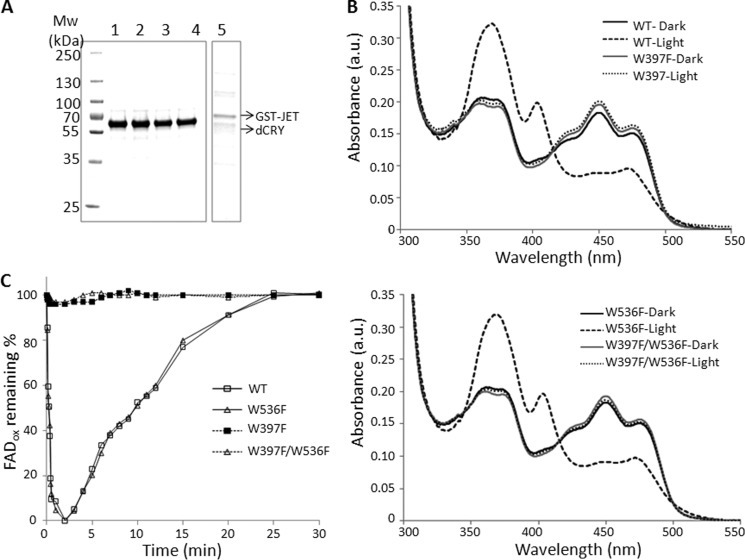
FIGURE 4.
Purification and properties of wild-type and mutant CRYs and GST-JET used in this study. Wild-type and mutant CRYs were expressed in Sf21 cells, and GST-JET was expressed in E. coli. Proteins were purified by affinity chromatography. A, purified proteins were separated on 4–15% SDS-PAGE and visualized by Coomassie Blue staining. Numbers on the left indicate size markers (Mw). Lane 1, WT-dCRY; lane 2, dCRY(W397F); lane 3, dCRY(W536F); lane 4, dCRY(W397F/W536F); lane 5, GST-JET. Each lane contains ∼2 μg of protein. B, dCRY photoreduction. Absorption spectra of wild-type and mutant dCRYs before and after light exposure (15 μmol m−2 s−1 for 30 min) are shown. a.u., absorbance units. C, photoreduction and reoxidation kinetics of wild-type and mutant dCRYs. The samples were exposed to 15 μmol m−2 s−1 light for 2 min and then kept in the dark under aerobic conditions, and the recovery of FADox was monitored by measuring changes in the absorbance at 450 nm.