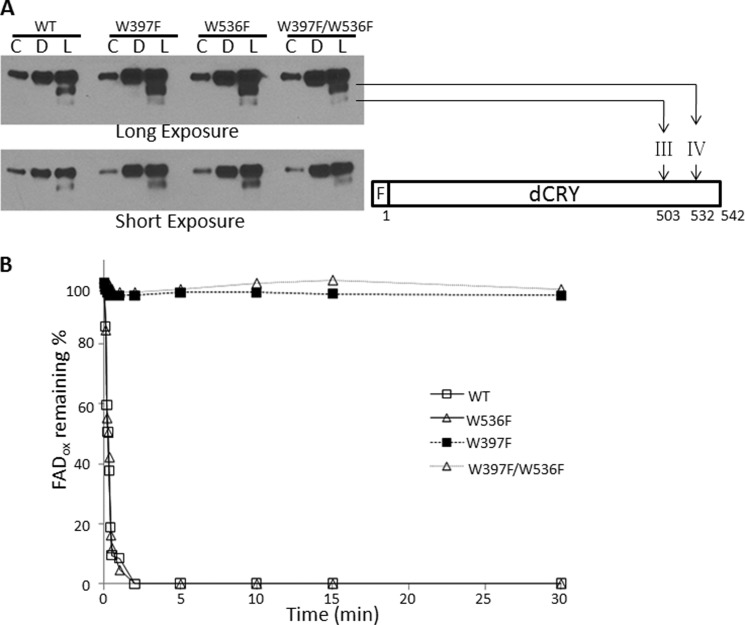
FIGURE 5.
Light-induced conformational change in the dCRY (FAD⨪) and dCRY (FADox). A, light-promoted trypsin proteolysis. dCRYs were kept in the dark (D) or exposed to light for 10 min (L) during treatment with trypsin; C indicates control with no trypsin (20-fold diluted sample was loaded as compared with trypsinized samples to get a nonsaturating signal). Then samples were separated on 4–15% SDS-PAGE, and then dCRY was detected by immunoblotting for the FLAG tag at the N terminus of the protein. The light-induced trypsin cleavage sites are indicated using the nomenclature employed previously for dCRY trypsin proteolysis (13). The FLAG tag at the N terminus is indicated with an F. B, wild-type or mutant dCRYs were exposed to 366-nm light (15 μmol m−2 s−1) as indicated, and the redox status of FAD was monitored by absorption at 450 nm.