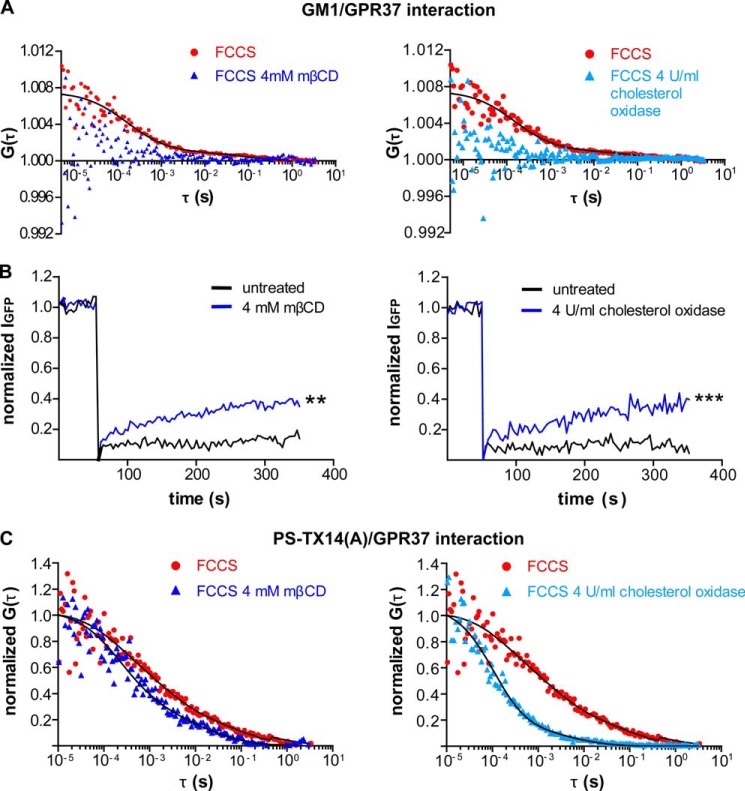
FIGURE 8.
Lipid rafts disruption with mβCD alters distribution and trafficking of GPR37, PS-TX14(A)TAMRA, and the complex between GPR37tGFP and PS-TX14(A)TAMRA at the plasma membrane. A, comparison of FCCS curves for GPR37tGFP and CTxB from untreated, mβCD-treated, or cholesterol oxidase-treated cells shows a significant cross-correlation (i.e. complex formation) between GPR37tGFP and CTxB in control cells with 85% of the complexes diffusing at τD1 = 0.5 ms and 15% at τD2 = 0.2 s. This cross-correlation was largely disrupted by mβCD and cholesterol oxidase. B, FRAP measurements for GPR37tGFP were performed on live cells (n = 23 cells for each treatment). Measurements were made on the same cell before and after treatment. Either mβCD or cholesterol oxidase increased GPR37tGFP fluorescence recovery compared with untreated cells, and the average amplitude of the FRAP curves at t = 350 s was significantly higher after mβCD or cholesterol oxidase treatment than before (**, p < 0.01; ***, p < 0.001, Student's t test). C, comparison of FCCS curves for GPR37tGFP and PS-TX14(A)TAMRA averaged from three separate untreated, mβCD-treated, or cholesterol oxidase-treated cells shows that cholesterol depletion shifts the curve toward shorter diffusion times. In untreated cells, 75 ± 15% of the complexes diffused at τD1 = 1.2 ± 0.6 ms and 25 ± 15% at τD2 = 330 ± 200 ms. In mβCD-treated cells, 80 ± 12% of the complexes diffused at τD1 = 550 ± 250 μs and 20 ± 12% at τD = 29 ± 15 ms. In cholesterol oxidase-treated cells, 89 ± 5% of the complexes diffused at τD1 = 170 ± 40 μs and 11 ± 5% at τD2 = 15 ± 9 ms.