FIGURE 2.
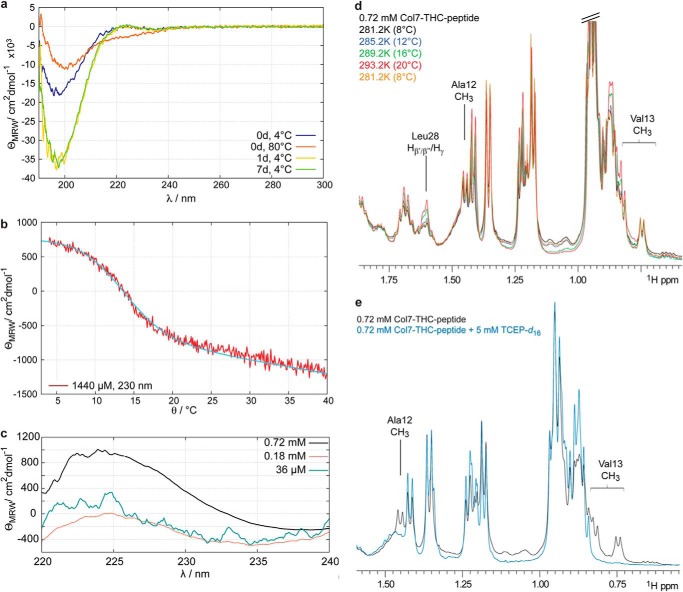
a, CD spectra of a 36 μm solution of Col7-THC peptide measured at different time points after sample preparation. Immediately after dissolution of the peptide, a CD spectrum is obtained that shows a negative band at ∼200 nm. Unfolding the peptide by increasing the temperature to 80 °C results in a CD spectrum that is typical for random coil structures. Triple helix formation is already achieved after incubation at 4 °C for 1 day. θMRW is the mean residue ellipticity. b, temperature-dependent changes of the CD signal at 230 nm allow the determination of the melting temperature of the collagen triple helix. The melting temperature has been determined to be 12.6 °C. c, formation of collagen triple helices is also concentration-dependent. At high peptide concentration a positive band at about 230 nm can be observed that is characteristic for collagen triple helix-forming peptides. d, detail of 500-MHz one-dimensional proton NMR spectra of a 0.72 mm peptide solution at different temperatures. Some resonances show temperature-dependent changes that are representative for unfolding of the triple helix (e.g. Leu-28). These changes are completely reversible (yellow curve). e, Val-13 and Ala-12 that are in between the two cysteine residues show two sets of resonances. Upon the addition of 5 mm TCEP-d16, which efficiently reduces disulfide bridges, one set of signals vanishes. These resonances are therefore sensitive to the formation of disulfide bridges.