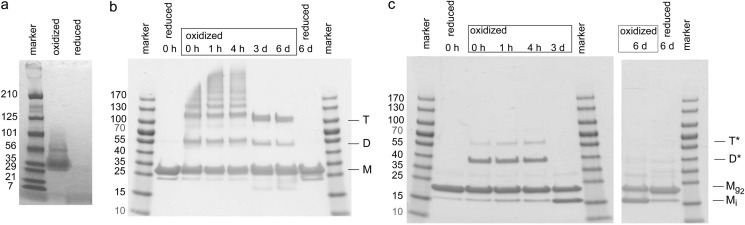
FIGURE 3.
a, SDS-PAGE (4–20%) analysis of a 1.4 mm solution of Col7-THC peptide. A major band is present for oxidative conditions but not for reducing conditions. The apparent molecular mass cannot be used to draw conclusions of the oligomerization state because peptides can have an unusual behavior in SDS-PAGE. b, SDS-PAGE (4–20%) analysis shows trimer formation of CysvWFA2-THC peptide. After the addition of glutathione, several additional bands at higher molecular mass are observed. The samples of day 3 and 6 show the presence of trimer (T), dimer (D), and monomer (M). Samples taken at 0 h and day 6 have also been analyzed under reducing conditions in the SDS-PAGE. CysvWFA2-THC peptide concentration was 10 μm, and sample loading is 7.5 μg of CysvWFA2-THC peptide per well. c, SDS-PAGE of a CysvWFA2 sample shows formation of oligomers upon the addition of oxidized glutathione (D*, T*), but after 3 days of incubation, only bands corresponding to monomers are present. The band of CysvWFA2 with an apparent molecular mass of 15 kDa represents the intramolecular disulfide bridge (Mi). Because the band at higher molecular mass is at the same height as the reduced proteins, this band is attributed to CysvWFA2 linked to two molecules of glutathione (Mg2). Oxidation was carried out with a 20 μm CysvWFA2 solution. 8 μg of CysvWFA2 are loaded per well.