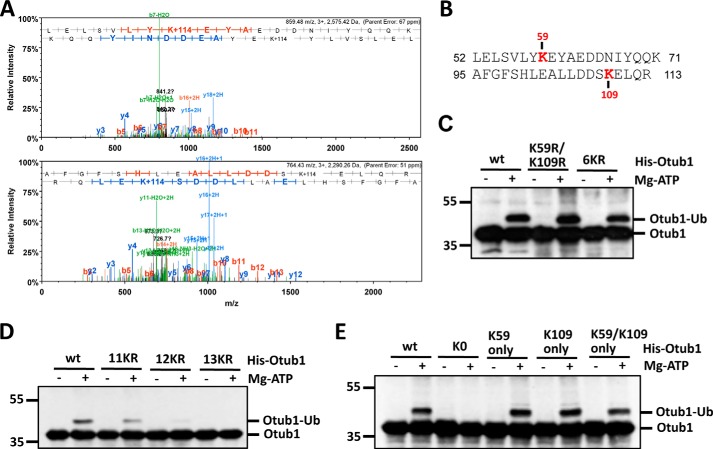
FIGURE 2.
Otub1 is monoubiquitinated by UbcH5 primarily at Lys-59 and Lys-109. A, identification of the monoubiquitination sites by mass spectrometry. The MS/MS spectrum (+3 ion) of Otub152–71 indicating ubiquitination at residue Lys-59 (top panel) and the MS/MS spectrum (+3 ion) of Otub195–113 indicating ubiquitination at residue Lys-109 (bottom panel) are shown. Matched b-series fragment ions are indicated in red, matched y-ion series fragment ions are indicated in blue, and matched fragment ions (both y and b) undergoing loss of water are indicated in green. B, summary of the peptides containing Ub-modified lysines (Lys-59 and Lys-109) identified by LC-MS/MS analysis. C and D, Otub1wt, Otub1K59R/K109R, Otub16KR, and Otub111KR, but not Otub112KR and Otub113KR, were monoubiquitinated by UbcH5 in vitro. WT Otub1 or its mutants, as indicated, were incubated with E1, E2, and Ub in the presence or absence of ATP, followed by IB analysis using anti-Otub1. E, Otub1wt, Otub1K59 only, Otub1K109 only, and Otub1K59/K109 only, but not Otub1K0, were monoubiquitinated by UbcH5 in vitro. WT Otub1 or its mutants, as indicated, were incubated with E1, E2, and Ub in the presence or absence of ATP, followed by IB analysis using anti-Otub1.