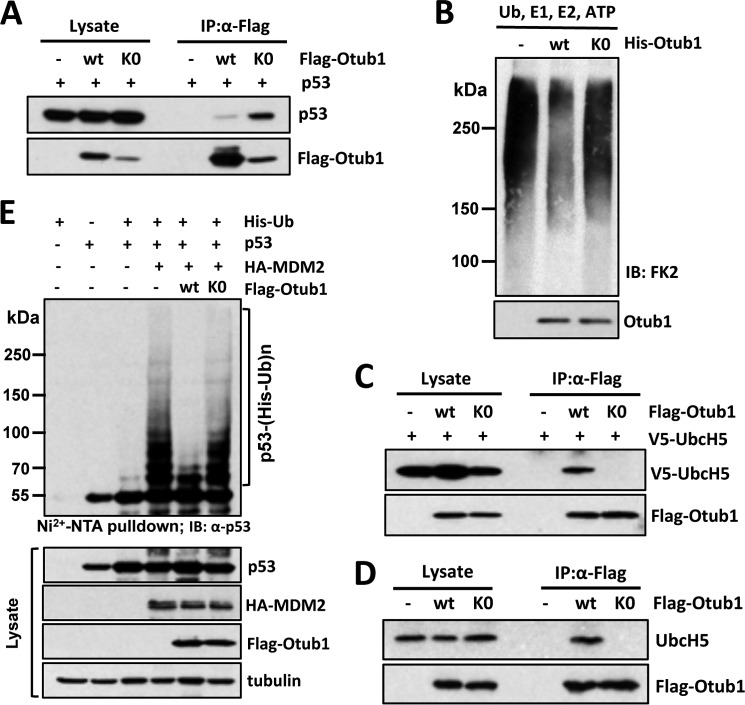
FIGURE 5.
Lysine-free Otub1 fails to interact with UbcH5 and suppress UbcH5 activity in vitro and MDM2-mediated p53 ubiquitination in cells. A, Otub1K0 binds to p53. H1299 cells transfected with p53 together with Otub1wt or Otub1K0 were subjected to co-IP using anti-FLAG antibodies, followed by IB analysis. B, Otub1K0 does not suppress UbcH5-dependent ubiquitin chain formation. The in vitro ubiquitination reactions were conducted in the presence of E1, E2, Ub, and ATP in the absence or presence of His-tagged Otub1wt or Otub1K0, as indicated. The reactions were assayed by IB analysis using anti-conjugated Ub antibody (clone FK2) (top panel). C and D, Otub1K0 does not interact with UbcH5 in cells. H1299 cells transfected with 0.25 μg of the FLAG-Otub1wt plasmid or 1.5 μg of the FLAG-Otub1K0 plasmid alone (D) or together with 1.5 μg of V5-UbcH5 (C) were subjected to co-IP with anti-FLAG antibodies, followed by IB analysis. E, Otub1K0 does not inhibit MDM2-mediated p53 ubiquitination in cells. H1299 cells transfected with indicated plasmids were subjected to pulldown using Ni-NTA beads under denaturing conditions, followed by IB analysis. The ubiquitinated species of p53 are indicated.