FIGURE 4.
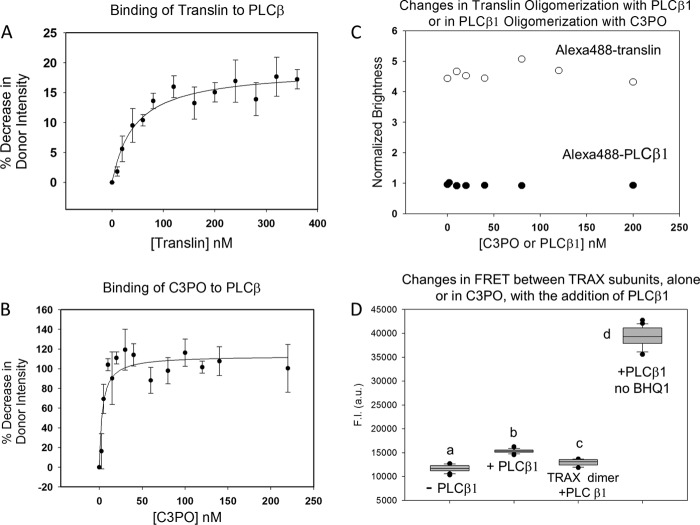
Association between PLCβ, translin, and C3PO. A, binding studies monitoring the increase in FRET as seen by the % decrease in donor intensity of 20 nm Alexa488-PLCβ1 with the addition of Alexa546-translin, n = 6; B, binding studies monitoring the decrease in fluorescence of 2 nm of Alexa488-PLCβ1 or β3 with the addition of BHQ1-C3PO, n = 9. Note that the concentrations of translin and C3PO refer to the total concentrations of the protein and do not refer to the concentration of protein octamers. C, changes in oligomerization Alexa488-translin as PLCβ1 is incrementally added or changes in oligomerization of Alexa488-PLCβ1 as C3PO is added where changes in oligomerization were monitored by brightness values derived from PCH analysis of FCS data, and where the values are relative to free Alexa488 (n = 4–8). The relative brightness of Alexa488-translin is lower than the expected value of 8, which we interpret as being due to the labeling ratio (∼0.6:1 Alexa488/translin). The diffusion coefficient is consistent with a higher order oligomer (i.e. D = 28 ± 2μ2/s) and does not significantly change with PLCβ1 binding (D = 30 ± 2μ2/s). These data support the idea that one PLCβ molecule associates to the translin complex. If a larger number of PLCβ bound to translin, a change in diffusion would have been observed. D, results showing that TRAX maintains a dimer alone or in C3PO with the addition of PLCβ1. Alexa488-TRAX is reconstituted with BHQ1-TRAX (to quench Alexa488 fluorescence) and translin at a 1:1:6 stoichiometry in the absence (a) or presence (b) of 120 nM PLCβ1 (n = 2,6). Also shown are the intensities for the Alexa488-TRAX/BHQ1-TRAX dimer (i.e. not in the context of C3PO) with PLCβ1 (c) or Alexa488-TRAX/translin (2:6) without quencher and with PLCβ1 (d) (n = 2). These results suggest that PLCβ does not displace a TRAX subunit from the TRAX dimer or C3PO octamer. fluorescence intensity (F.I.). a.u., arbitrary units.