FIGURE 1.
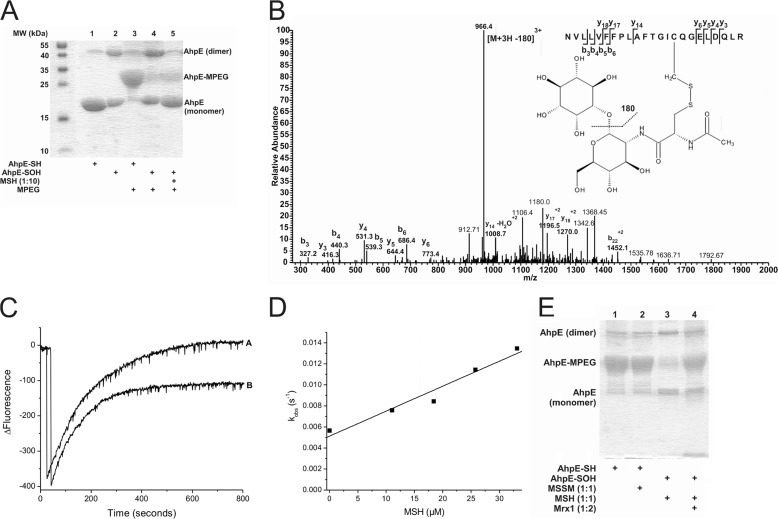
Oxidized MtAhpE reacts with MSH forming a mixed disulfide that is reduced by MtMrx1. A, alkylation of MtAhpE with PEG-maleimide. Reduced or oxidized MtAhpE (20 μm) was incubated with or without MSH (200 μm). Proteins were precipitated with TCA, treated with PEG-maleimide (5 mm), and evaluated on a CBB-stained 15% SDS-PAGE. B, identification of S-mycothiolation on cysteine 45 of MtAhpE. Sample was obtained by adding MSH (60 μm) to oxidized MtAhpE (20 μm). The LC-MS/MS spectrum shows data obtained from a 3+ parent ion with m/z = 1026.5. The spectrum displays one major daughter ion at m/z 966.4 corresponding to the neutral loss of inositol (180 Da) after fragmentation at a C-O bond. The y- and b- series of ions allowed exact localization of the mixed disulfide between mycothiol and the cysteine residue. C, kinetics of reaction of oxidized MtAhpE with MSH. Time-dependent decrease (oxidation) and increase (overoxidation) in the intrinsic fluorescence intensity (λex = 295 nm, λem = 340 nm) of MtAhpE (2 μm) in the absence (A) or presence (B) of MSH (18 μm) upon addition of H2O2 (150 μm) in 100 mm sodium phosphate buffer plus 0.1 mm DTPA. D, effect of the MSH concentration on the observed rate constants of MtAhpE intrinsic fluorescence change caused by overoxidation. E, MtAhpE-SS-M reduction by MtMrx1. Reduced (lanes 1 and 2) or oxidized (lanes 3 and 4) MtAhpE (20 μm) was incubated with MSSM (lane 2) or MSH (lanes 3 and 4)(20 μm) for 30 min, followed by incubation without (lane 3) or with MtMrx1 (lane 4) (20 μm) for 15 min. Samples were treated with PEG-maleimide (5 mm) and evaluated by CBB-stained 15% SDS-PAGE.