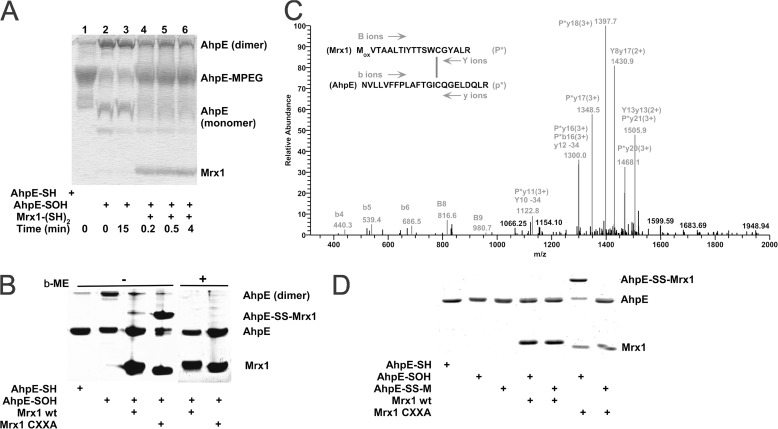
FIGURE 2.
MtAhpE is reduced by wild type MtMrx1 by a dithiolic mechanism. A, reduced (lane 1) or oxidized (lanes 2–6) MtAhpE (10 μm) incubated in the absence (lanes 1–3) or presence of reduced MtMrx1 (16 μm) for indicated times (lanes 4–6) and treated with 5 mm PEG-maleimide were evaluated on a Coomassie Brilliant Blue (CBB) stained 15% SDS-PAGE. B, reduced and oxidized MtAhpE alone (10 μm), or oxidized MtAhpE incubated with MtMrx1 wt or MtMrx1 CXXA (30 μm) for 15 min were evaluated on a CBB-stained 15% SDS-PAGE in the absence (lanes 1–4, respectively) or presence (lanes 4–8, respectively) of β-ME. A novel band with a molecular mass compatible with a mixed disulfide formation between MtAhpE and MtMrx1CXXA is indicated as MtAhpE-SS-MtMrx1. C, mass spectrometric analysis of the MtAhpE-SS-MtMrx1 complex is shown in Fig. 2B. A quadruply charged parent ion of [M+4H]4+ = 1183.7 Da shows fragmentation characteristics of a disulfide linkage between Cys17 of MtMrx1 and Cys45 of MtAhpE, as determined by the DBond software (29). P*, one strand of a dipeptide; p*, the other strand of a dipeptide; capital letters, fragment ions from peptide P*; lowercase letters, fragment ions from peptide p*. The loss of 34 atomic mass units represents formation of dehydroalanine from C-S bond fragmentation. D, reaction of MtAhpE-SS-M with MtMrx1CXXA does not form a protein-protein intermolecular mixed disulfide. Reduced (lane 1) and oxidized MtAhpE (lanes 2–7) alone (lanes 2, 4, and 6), or incubated with MSH during 30 min (lanes 3, 5, and 7), were incubated in the absence (lanes 2 and 3) or presence of MtMrx1 wt (lanes 4 and 5) or MtMrx1CXXA (lanes 6 and 7) for 15 min. Reaction was stopped by addition of 5 mm NEM, and samples were evaluated on a CBB-stained 15% SDS-PAGE under non-reducing conditions. A novel band with a molecular mass compatible with a mixed disulfide formation between MtAhpE and MtMrx1CXXA is indicated as MtAhpE-SS-MtMrx1.