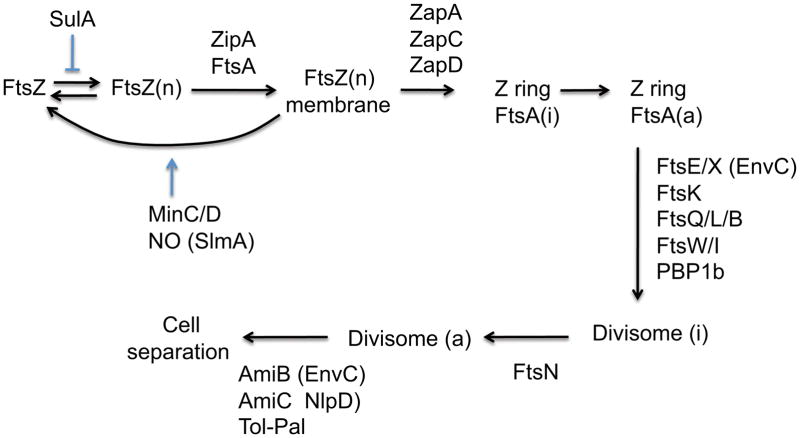
Fig. 4.
Diagram of assembly of the Z ring and maturation to the divisome in E. coli. FtsZ polymers (FtsZ[n]) are tethered to the membrane by FtsA and ZipA, which leads to formation of the Z ring. However, FtsA is not immediately available to recruit downstream proteins (FtsA[i]). Although not essential, several FtsZ interacting proteins (ZapA–D) localize to the ring and promote the integrity of the Z ring. Antagonists of FtsZ assembly, MinC/D and SlmA, are positioned away from midcell so that it is permissive for Z ring formation. When enough monomeric FtsA (active [a]) is present at the Z ring the remaining Fts proteins and PBP1b are recruited. The arrival of FtsN signals the divisome is complete and activation of septal PG synthesis occurs (divisome [a]). The septal cross wall is split by AmiB, which is activated by EnvC (recruited earlier), and AmiC, which is activated by NlpD. Although EnvC is recruited early, the arrival of AmiC, AmiB and NlpD depend upon the start of septal PG synthesis induced by the arrival of FtsN. The Tol-Pal complex is needed for efficient invagination of the outer membrane.