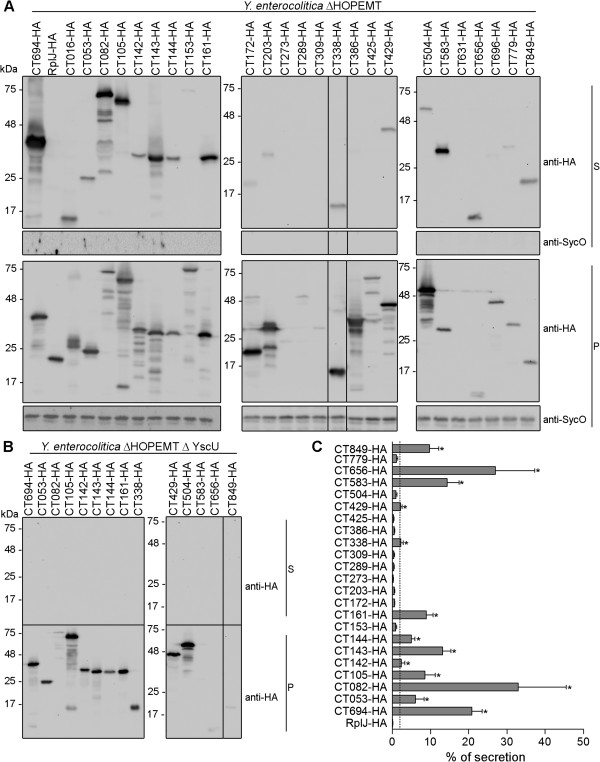
Figure 3.
Analysis of the T3S of C. trachomatis full-length proteins by Y. enterocolitica. Y. enterocolitica T3S-proficient (ΔHOPEMT) (A) and T3S-defective (ΔHOPEMT ΔYscU) (B) were used to analyze secretion of full-length C. trachomatis proteins with a C-terminal HA epitope tag. Immunoblots show the result of T3S assays in which proteins in culture supernatants (S, secreted proteins) and in bacterial pellets (P, non-secreted proteins) from ~5 x 108 and ~5 x 107 bacteria, respectively, were loaded per lane. The known C. trachomatis T3S substrates CT082 [26,27] and CT694 [14] were used as positive controls, and the C. trachomatis ribosomal protein RplJ was used as a negative control. SycO is a strictly cytosolic Yersinia T3S chaperone [44,51] and its immunodetection ensured that the presence of HA-tagged proteins in the culture supernatants was not a result of bacterial lysis or contamination. (C) The percentage (%) of secretion of each protein by Y. enterocolitica ΔHOPEMT was calculated by densitometry, as the ratio between the amount of secreted and total protein. The threshold to decide whether a protein was secreted was set to 2% (dashed line), based on the % of secretion of RplJ-HA. Data are the mean ± SEM from at least 3 independent experiments.