Abstract
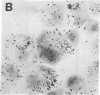
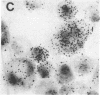
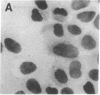
The sensitivity of in situ hybridization has been increased at least 10-fold by hybridizing in cDNA excess, by increasing the diffusion of the cDNA through the cells, by hybridizing at optimum temperature, and by stabilizing hybrids during autoradiography. Saturation of intracellular RNA with [3H]cDNA has been achieved. The assay is quantitative. In situ hybridization has been used to detect and quantitate visna virus RNA in infected cells. By using [3H]cDNA with specific activity of 2 X 10(8) dpm/micrograms and conditions that reduce background to negligible levels, 10--20 copies of viral RNA per cell can be detected and quantitated after 2 days of autoradiographic exposure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnstiel M. L., Sells B. H., Purdom I. F. Kinetic complexity of RNA molecules. J Mol Biol. 1972 Jan 14;63(1):21–39. doi: 10.1016/0022-2836(72)90519-0. [DOI] [PubMed] [Google Scholar]
- Bonner J. J., Pardue M. L. Ecdysone-stimulated RNA synthesis in imaginal discs of Drosophila melanogaster. Assay by in situ hybridization. Chromosoma. 1976 Oct 12;58(1):87–99. doi: 10.1007/BF00293443. [DOI] [PubMed] [Google Scholar]
- Brahic M., Filippi P., Vigne R., Haase A. T. Visna virus RNA synthesis. J Virol. 1977 Oct;24(1):74–81. doi: 10.1128/jvi.24.1.74-81.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M. The infection caused by visna virus. A model for the study of slow degenerative diseases of the central nervous system. Pathol Biol (Paris) 1977 Jun;25(6):365–368. [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Birfelder E. J., Sanders J. P., Borst P. DNA-DNA hybridization on nitrocellulose filters. 1. General considerations and non-ideal kinetics. Eur J Biochem. 1974 Sep 16;47(3):535–543. doi: 10.1111/j.1432-1033.1974.tb03722.x. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Narayan P., Griffin D., Price D. Slow persistent infection caused by visna virus: role of host restriction. Science. 1977 Jan 14;195(4274):175–177. doi: 10.1126/science.188133. [DOI] [PubMed] [Google Scholar]
- Haase A. T. The slow infection caused by visna virus. Curr Top Microbiol Immunol. 1975;72:101–156. doi: 10.1007/978-3-642-66289-8_4. [DOI] [PubMed] [Google Scholar]
- Harrison P. R., Conkie D., Affara N., Paul J. In situ localization of globin messenger RNA formation. I. During mouse fetal liver development. J Cell Biol. 1974 Nov;63(2 Pt 1):402–413. doi: 10.1083/jcb.63.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. R., Wetmur J. G. Effect of chemical modification on the rate of renaturation of deoxyribonucleic acid. Deaminated and glyoxalated deoxyribonucleic acid. Biochemistry. 1973 Jan 30;12(3):558–563. doi: 10.1021/bi00727a032. [DOI] [PubMed] [Google Scholar]
- Imaizumi T., Diggelmann H., Scherrer K. Demonstration of globin messenger sequences in giant nuclear precursors of messenger RNA of avian erythroblasts. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1122–1126. doi: 10.1073/pnas.70.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Moar M. H., Purdom I. F., Jones K. W. Influence of temperature on the detectibility and chromosomal distribution of specific DNA sequences by in situ hybridisation. Chromosoma. 1975 Dec 29;53(4):345–359. doi: 10.1007/BF00294082. [DOI] [PubMed] [Google Scholar]
- Monahan J. J., McReynolds L. A., O'Malley B. W. The ovalbumin gene. In vitro enzymatic synthesis and characterization. J Biol Chem. 1976 Dec 10;251(23):7355–7362. [PubMed] [Google Scholar]
- Szabo P., Elder R., Steffensen D. M., Uhlenbeck O. C. Quantitative in situ hybridization of ribosomal RNA species to polytene chromosomes of Drosophila melanogaster. J Mol Biol. 1977 Sep 25;115(3):539–563. doi: 10.1016/0022-2836(77)90170-x. [DOI] [PubMed] [Google Scholar]