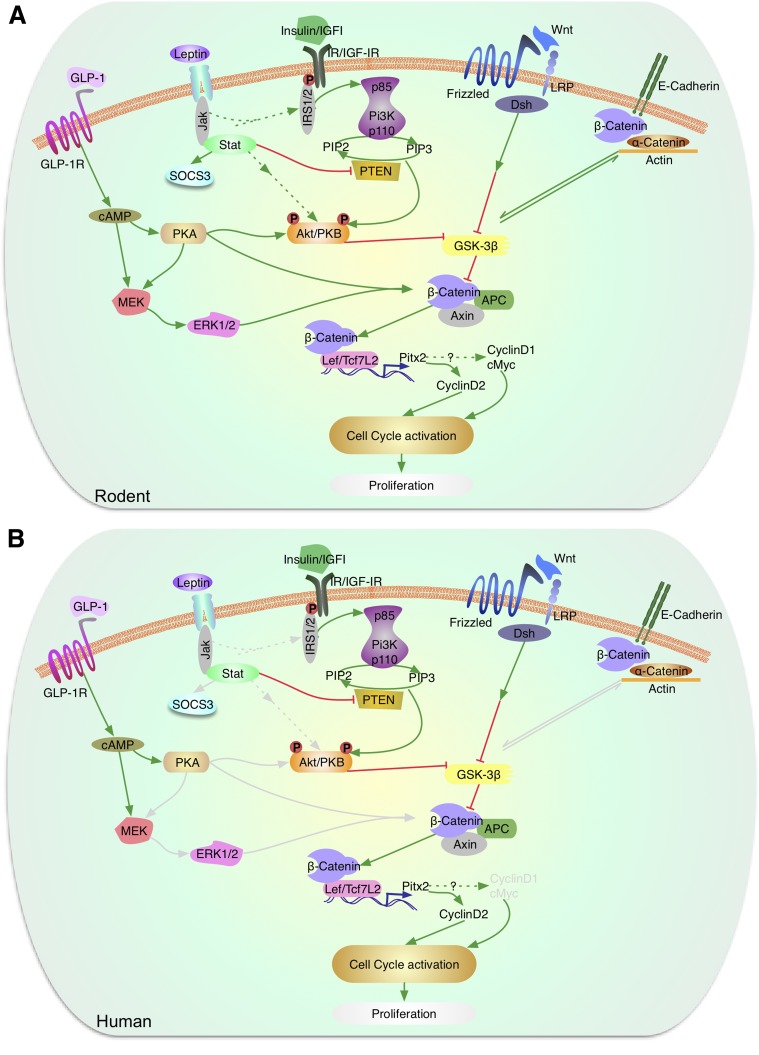
Figure 3.
Signaling by leptin, Wnt, and β-catenin in the regulation of β-cell proliferation. A: In murine models, leptin acts via the JAK-STAT pathway to inhibit PTEN and also modulate Akt/PKB and p70S6k. Akt/PKB, which is also activated by growth factor (insulin/IGF-1) signaling, modulates GSK3β. The Wnt/frizzled pathway also regulates GSK3β, which blocks phosphorylation of β-catenin to control the expression of Lef/Tcf7L2 and cyclin D2 and potentially cyclin D1 and cMyc to activate the cell cycle and regulate proliferation. GLP-1 receptor signaling activated by GLP-1 or exendin-4 leads to elevation of cAMP and activation of protein kinase A, which can directly or indirectly via the MEK/ERK1/2 pathway phosphorylate β-catenin. Activation of the insulin or IGF-1 receptors leads to phosphorylation of serine/threonine residues in IRS2, activation of PI3K and Akt/PKB, which can, in turn, phosphorylate and inactivate GSK3β. B: In human β-cells, leptin receptors, GLP-1 receptors, and elements of the Wnt signaling pathway have been reported. However, the downstream proteins (marked in gray) that link to the proliferation response are not fully understood. (A high-quality color representation of this figure is available in the online issue.)