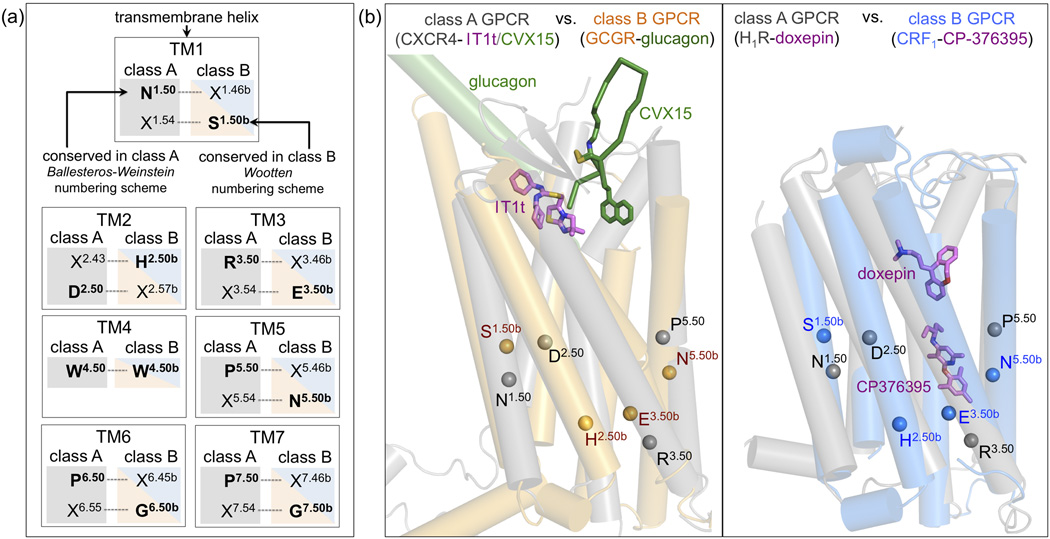
Figure 3.
The spatial correspondence between residues in TM helices of class A and class B GPCRs makes it possible to align the most conserved residues in class A (designated X.50, Ballesteros-Weinstein numbering) and class B (designated X.50b, Wootten numbering) for comparisons between GPCR classes (Box 1). (b) Structural alignment of CRF1 (blue) and GCGR (orange) to two representative class A GPCRs, H1R (PDB ID 3RZE) and CXCR4 (PDB IDs 3ODU/3OE0) (in grey). Helices are depicted as cylinders, ligands glucagon (GCGR), CP-376395 (CRF1), doxepin (H1R), and IT1t and CVX15 (CXCR4) as sticks. The location of the Cα-atoms of the most conserved residues of TM1–3 and TM5 among class A and class B GPCRs (Box 1) are indicated by spheres (TM4 is not depicted for clarity).