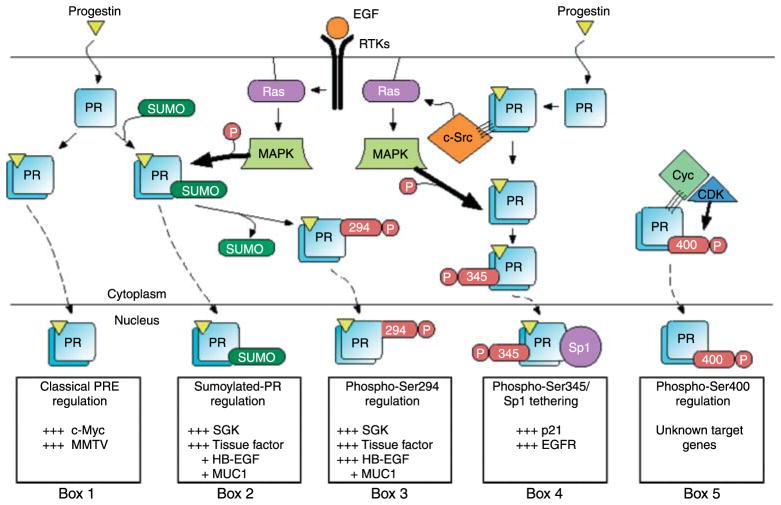
Figure 3.
Post-translational modification of PR results in the regulation of specific subpopulations of PR genes. A subset of PR is sumoylated on Lys388 in response to progestin binding. Cells respond to growth factors (e.g. EGF) by activation of MAPK signaling resulting in PR phosphorylation at Ser294 and desumoylation at Lys388. These signaling mechanisms provide three different post-translationally modified populations of PR that can activate gene transcription at selected promoters (Boxes 1–3). Additionally, progestin treatment results in PR-mediated activation of c-Src through direct interaction of PR’s proline-rich motif with c-Src’s SH3 domain (Boonyaratanakornkit et al. 2001). Progestin-induced c-Src activation results in MAPK signaling and phosphorylation at PR Ser345 (Faivre et al. 2008). Phosphorylated PR at Ser345 can tether to Sp1 and activate transcription at promoters with Sp1-binding sites (Box 4). PR interactions with CDK2 result in increased liganded and unliganded PR activity on unknown PR target-gene promoters (Box 5; Pierson-Mullany & Lange 2004). Many additional genes are likely to be differentially regulated by post-translationally modified PR transcription factors.