Abstract
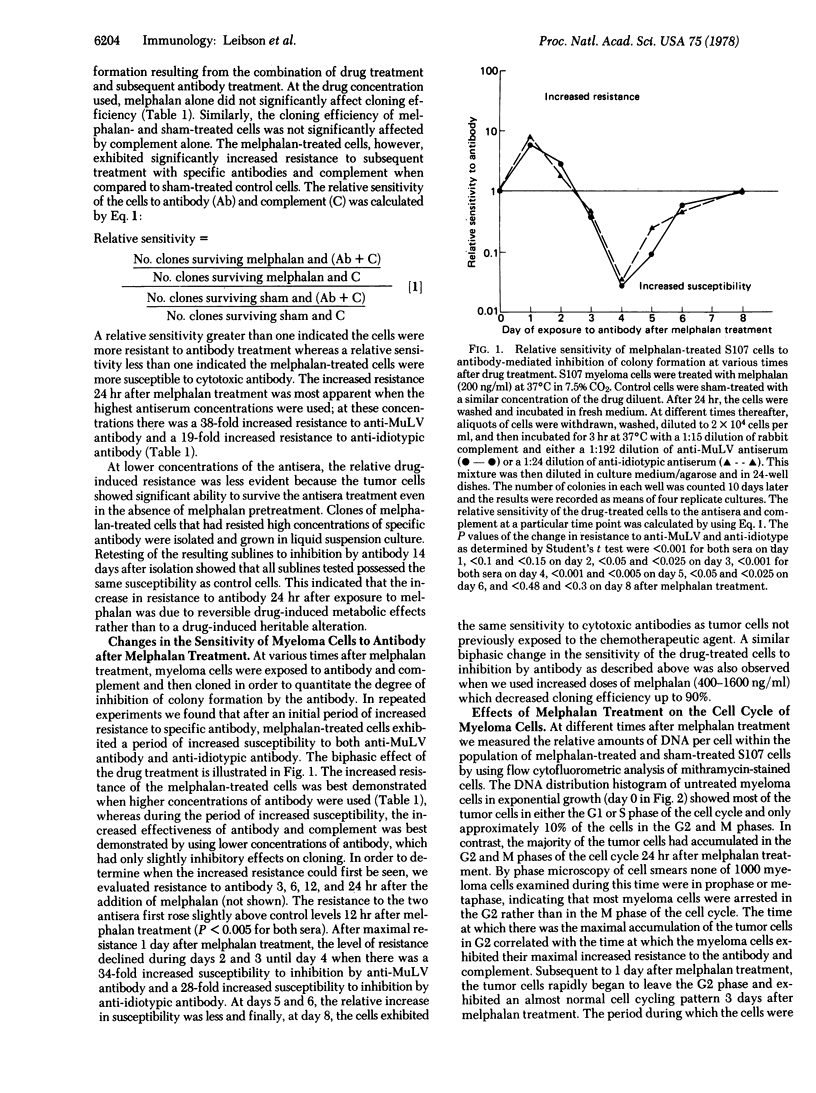
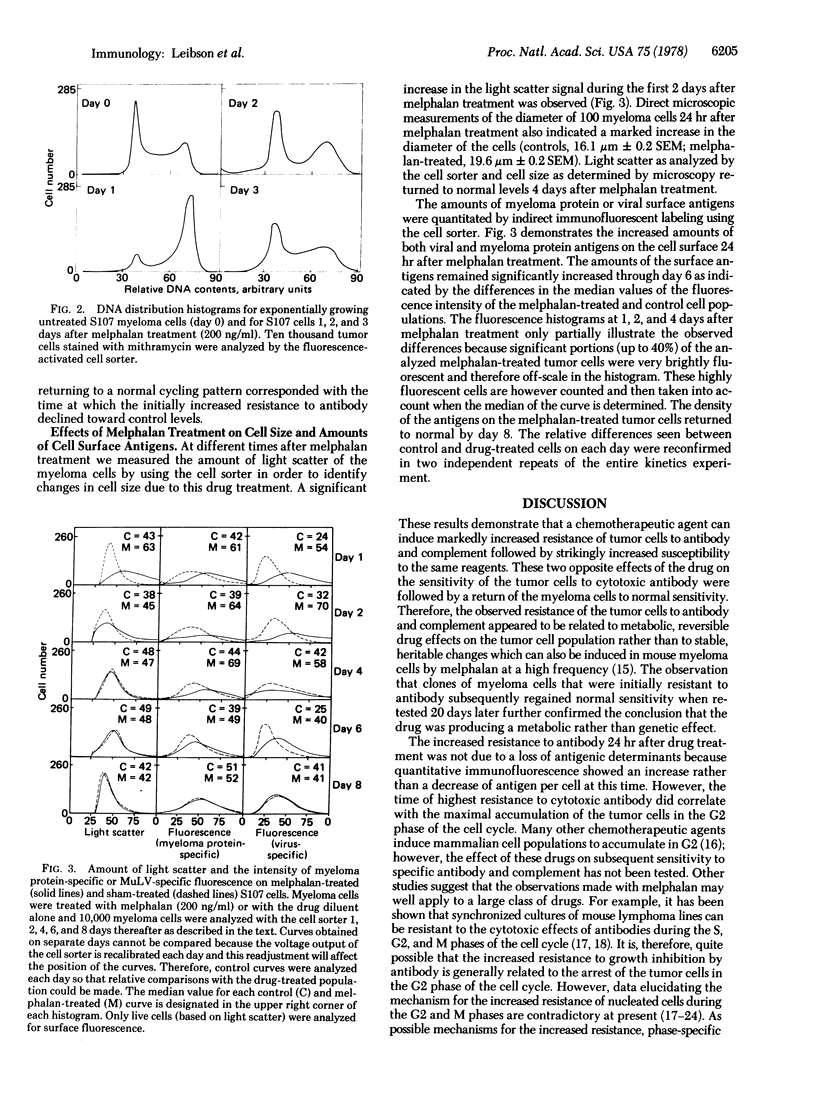
We report that a chemotherapeutic agent (melphalan) can affect the sensitivity of tumor cells to cytotoxic antibody. Depending on the time interval between drug treatment and subsequent exposure to antibody and complement, the tumor cells can be either more resistant or more susceptible to antibody when compared to control cells. The number of tumor cells surviving the combined treatment was determined by a colony inhibition assay. The two antisera used in this study were directed against either virus-specific or myeloma protein-specific antigens on the surface of S107 murine myeloma cells; identical results were obtained with both sera. Twenty-four hours after exposure to the drug, the number of tumor cells surviving the antibody treatment increased. During this period of increased resistance, the tumor cells were temporarily arrested in the G2 phase of the cell cycle. After this period of maximal resistance, the effect of cytotoxic antibody on the cells changed such that 4 days after melphalan treatment the cells were significantly more susceptible to the antibody than were the sham-treated control cells. The period of increased susceptibility correlated with an increased density of S107 myeloma protein and viral antigens on the surface of the tumor cells. Eight days after the drug treatment, the susceptibility of the tumor cells and the density of surface antigens both returned to normal levels. This study shows that the correct time interval between exposure to a drug and subsequent treatment with antibody is critical for maximal killing of the tumor cells. The basis for the differential sensitivity of the tumor cells to anti-body may be related to the drug-induced changes in the cell cycle and in antigen expression on the cell surface.
Keywords: melphalan, myeloma cells, cell-surface antigens, cell cycle, fluorescence-activated cell sorter
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle M. D., Ohanian S. H., Borsos T. Studies on the terminal stages of antibody-complement-mediated killing of a tumor cell. I. Evidence for the existence of an intermediate, T. J Immunol. 1976 May;116(5):1272–1275. [PubMed] [Google Scholar]
- Burakoff S. J., Martz E., Benacerraf B. Is the primary complement lesion insufficient for lysis? Failure of cells damaged under osmotic protection to lyse in EDTA or at low temperature after removal of osmotic protection. Clin Immunol Immunopathol. 1975 May;4(1):108–126. doi: 10.1016/0090-1229(75)90045-8. [DOI] [PubMed] [Google Scholar]
- Burk K. H., Drewinko B., Lichtiger B., Trujillo J. M. Cell cycle dependency of human sarcoma-associated tumor antigen expression. Cancer Res. 1976 Apr;36(4):1278–1283. [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Cikes M., Friberg S., Jr Expression of H-2 and Moloney leukemia virus-determined cell-surface antigens in synchronized cultures of a mouse cell line. Proc Natl Acad Sci U S A. 1971 Mar;68(3):566–569. doi: 10.1073/pnas.68.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P., Baumal R., Laskov R., Scharff M. D. Cloning of mouse myeloma cells and detection of rare variants. J Cell Physiol. 1972 Jun;79(3):429–440. doi: 10.1002/jcp.1040790313. [DOI] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Cooper N. R., Polley M. J., Oldstone M. B. Failure of terminal complement components to induce lysis of Moloney virus transformed lymphocytes. J Immunol. 1974 Feb;112(2):866–868. [PubMed] [Google Scholar]
- Crissman H. A., Tobey R. A. Cell-cycle analysis in 20 minutes. Science. 1974 Jun 21;184(4143):1297–1298. doi: 10.1126/science.184.4143.1297. [DOI] [PubMed] [Google Scholar]
- Dean P. N., Jett J. H. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974 Feb;60(2):523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson L. K., Plocinik B. A., Rogentine G. N., Jr HL-A expression on the G1, S, and G2 cell-cycle stages of human lymphoid cells. J Natl Cancer Inst. 1974 Oct;53(4):913–920. doi: 10.1093/jnci/53.4.913. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Ishizaka K. A simplified procedure for the preparation of immunoglobulin-class-specific antisera. J Immunol. 1969 Jul;103(1):56–61. [PubMed] [Google Scholar]
- Lerner R. A., Oldstone M. B., Cooper N. R. Cell cycle-dependent immune lysis of Moloney virus-transformed lymphocytes: presence of viral antigen, accessibility to antibody, and complement activation. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2584–2588. doi: 10.1073/pnas.68.10.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman R., Potter M., Mushinski E. B., Humphrey W., Jr, Rudikoff S. Genetics of a new IgVH (T15 idiotype) marker in the mouse regulating natural antibody to phosphorylcholine. J Exp Med. 1974 Apr 1;139(4):983–1001. doi: 10.1084/jem.139.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panem S., Schauf V. Cell-cycle dependent appearance of murine leukemia-sarcoma virus antigens. J Virol. 1974 Jun;13(6):1169–1175. doi: 10.1128/jvi.13.6.1169-1175.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Cooper N. R., Dierich M. P., Reisfeld R. A. Variation in susceptibility of a human lymphoid cell line to immune lysis during the cell cycle. Lack of correlation with antigen density and complement binding. J Exp Med. 1974 Aug 1;140(2):578–590. doi: 10.1084/jem.140.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prend'homme J. L., Buxbaum J., Scharff M. D. Mutagenesis of mouse myeloma cells with 'Melphalan'. Nature. 1973 Oct 12;245(5424):320–322. doi: 10.1038/245320a0. [DOI] [PubMed] [Google Scholar]
- Schreiber H., Leibson P. Suppression of myeloma growth in vitro by anti-idiotypic antibodies: inhibition of DNA synthesis and colony formation. J Natl Cancer Inst. 1978 Jan;60(1):225–233. doi: 10.1093/jnci/60.1.225. [DOI] [PubMed] [Google Scholar]
- Segerling M., Ohanian S. H., Borsos T. Chemotherapeutic drugs increase killing of tumor cells by antibody and complement. Science. 1975 Apr 4;188(4183):55–57. doi: 10.1126/science.46622. [DOI] [PubMed] [Google Scholar]
- Shin H. S., Economou J. S., Pasternack G. R., Johnson R. J., Hayden M. L. Antibody-mediated suppression of grafted lymphoma. IV. Influence of time of tumor residency in vivo and tumor size upon the effectiveness of suppression by syngeneic antibody. J Exp Med. 1976 Nov 2;144(5):1274–1283. doi: 10.1084/jem.144.5.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey R. A., Crissman H. A. Use of flow microfluorometry in detailed analysis of effects of chemical agents on cell cycle progression. Cancer Res. 1972 Dec;32(12):2726–2732. [PubMed] [Google Scholar]
- Tobey R. A. Different drugs arrest cells at a number of distinct stages in G2. Nature. 1975 Mar 20;254(5497):245–247. doi: 10.1038/254245a0. [DOI] [PubMed] [Google Scholar]
- WINN H. J. Immune mechanisms in homotransplantation. I. The role of serum antibody and complement in the neutralization of lymphoma cells. J Immunol. 1960 May;84:530–538. [PubMed] [Google Scholar]
- Watson J., Ralph P., Sarkar S., Cohn M. Leukemia viruses associated with mouse myeloma cells. Proc Natl Acad Sci U S A. 1970 Jun;66(2):344–351. doi: 10.1073/pnas.66.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler G. P., Bowdon B. J., Adamson D. J., Vail M. H. Comparison of the effects of several inhibitors of the synthesis of nucleic acids upon the viability and progression through the cell cycle of cultured H. Ep. no. 2 cells. Cancer Res. 1972 Dec;32(12):2661–2669. [PubMed] [Google Scholar]


