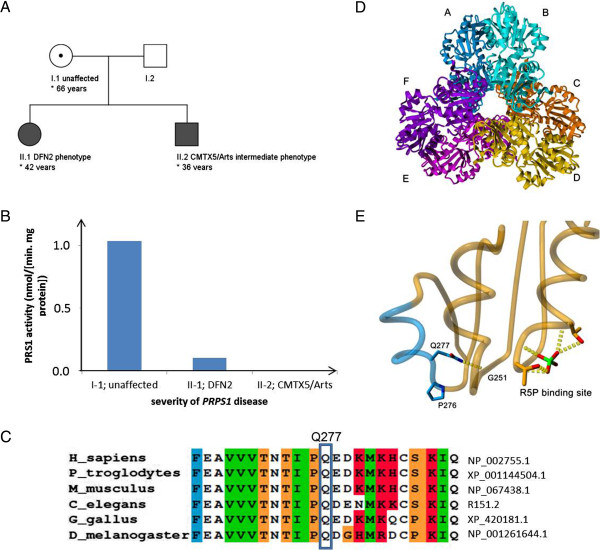
Figure 1.
Pedigree, PRS enzymatic activity, conservation, and predicted protein structural effects of the novel Gln277Pro PRPS1 variant. (A) Pedigree of the family shows maternal inheritance of the PRPS1 mutation with variable phenotypic severity of disease, reaching from an unaffected state in the mother (I-1) to a mildly affected DFN2 phenotype in the sister of the index patient (II-1) to a severe Arts/CMTX5 overlap syndrome in the index patient (II-2). (B) The severity of the phenotype correlated with the PRS1 enzymatic activity in erythrocytes. (C) Protein alignment demonstrates high conservation for the mutated amino acid position 277 in the PRPS1 protein across mammals and non-mammals. (D) PRS1 catalyses the synthesis of phosphoribosylpyrophosphate (PRPP) from ribose 5-phosphate (R5P) and ATP, and is thought to be physiologically functional as a hexamer, consisting of three homodimers arranged in a propeller-like shape. In this hexamer, the N-terminal domains are located at the centre and the C-terminal domains at the outside. (E) Gln277 is predicted to interact with Gly251, which is close proximity to the R5P binding site The p.Gln277Pro change will result in a loss of this hydrogen bonding interaction potentially destabilizing the region surrounding the R5P binding site, thus affecting the PRPS1 catalytic active site.